Thema: Baytril. ?
AW: Baytril. ?
Antibiotikum (Gyrasehemmer) fьr Hunde und Katzen
1 ml enthдlt 25 mg Enrofloxacin, Kalii hydroxidum, Alcohol butylicus, Aqua ad Iniectabilia qs ad 1 ml.
Enrofloxacin gehцrt zur chemischen Klasse der Fluorochinolone. Die Substanz besitzt eine bakterizide Wirkung, die ьber eine Bindung an die A-Untereinheit der bakteriellen DNA-Gyrase und die dadurch verursachte selektive Hemmung dieses Enzyms vermittelt wird. Die DNA-Gyrase gehцrt zu den Topoisomerasen, die bei Bakterien an der Replikation, Transkription und Rekombination der DNA beteiligt sind. Fluorochinolone beeinflussen auch Bakterien in der Ruhephase aufgrund von Дnderungen der Zellwandpermeabilitдt. Diese Mechanismen erklдren, warum die Lebensfдhigkeit der Bakterien bei Einwirkung von Enrofloxacin sehr schnell nachlдsst. Bei Enrofloxacin liegen die inhibitorischen und die bakteriziden Konzentrationen dicht nebeneinander. Sie
Aufgrund des Wirkungsmechanismus erfolgt eine Verminderung der Empfindlichkeit der aufgefьhrten Bakterien stufenweise und dementsprechend langsam (Multi-Step-Typ).
Nach s.c. und i.m. Verabreichung von Enrofloxacin werden bereits nach 1 - 2 Stunden maximale Wirkstoffspiegel in Plasma und Geweben erreicht. Enrofloxacin besitzt ein grosses Verteilungsvolumen. Die Konzentrationen in den Geweben und den Organen ьbertreffen zumeist die Plasmaspiegel deutlich. Nach vorschriftsgemдsser Dosierung wird die minimale Hemmkonzentration relevanter Erreger im Plasma und in verschiedenen Zielgeweben wдhrend mehrerer Stunden ьberschritten. Organe, in denen hohe Konzentrationen erwartet werden kцnnen, sind beispielsweise Lunge, Leber, Niere, Harnblase, Prostata, Gebдrmutter, Haut, Knochen und lymphatisches Gewebe.
Aufgrund seines umfassenden Wirkungsspektrums kann Baytril bei allen bakteriellen Einzel- und Mischinfektionen sowie Mykoplasmosen, insbesondere der Atmungs- und Verdauungsorgane, des Harn und Geschlechtsapparates, der Haut, des дusseren Ohres sowie von Wunden eingesetzt werden.
0,2 ml pro 1 kg Kцrpergewicht (5 mg/kg KGW)
Art und Dauer der Anwendung
Zur subkutanen Injektion.
Die Behandlung erfolgt an 5 aufeinanderfolgenden Tagen, bei chronischen und schwer verlaufenden Erkrankungen an bis zu 10 Tagen. Die empfohlenen Dosierungen sollten nicht ьberschritten werden.
Nicht anwenden bei:
- bereits besteheneden Knorpelwachstumsstцrungen
- Trдchtige und in der Stillperiode stehende Tiere sind von der Behandlung auszuschliessen.
- Tiere mit zentralen Anfallsleiden.
- Vorliegen von Resistenz gegen Chinolonen, da gegenьber diesen eine nahezu vollstдndige, gegenьber anderen Fluorochinolonen eine komplette Kreuztesistenz besteht.
- Die Ausscheidung von Enrofloxacin erfolgt zum Teil ьber die Niere, bei bestehenden Nierenschдden ist daher wie bei allen Fluorochinolonen mit einer Verlдngerung der Ausscheidung zu rechnen.
- Nicht bei Tieren anwenden, die der Gewinnung von Lebensmitteln dienen.
Hunde unter einem Jahr sind vor der Behandlung auszuschliessen, da wдhrend der Phase des intensiven Wachstums, artspezifisch bei grosswьchsigen Hunderassen, Gelenkknorpelschдden auftreten kцnnen. Aus Sicherheitsgrьnden wird bei sehr grossen Hunderassen wegen der lдngeren Wachstumsphase ein Ausschluss von bis zu 18 Monaten empfohlen.
Pharmacovigilance: Meldung erstatten
In seltenen Fдllen kann es bei Katzen, die hohe Dosierungen erhalten (20 mg/kg Kцrpergewicht und darьber), zu Sehstцrungen kommen.
Hunde und Katzen:
- Vereinzelt gastrointestinale Stцrungen.
- In seltenen Fдllen kann es zu Reaktionen an der Injektionsstelle kommen.
- Die Elimination von Theophyllin kann verzцgert werden.
- Bei Kombination von Baytril (Enrofloxacin) mit Chloramphenicol, Makrolid-Antibiotika oder Tetrazyklinen kцnnen antagonistische Effekte auftreten.
Nach Ablauf des Verfalldatums nicht mehr anwenden. Arzneimittel fьr Kinder unzugдnglich aufbewahren. Aufbrauchfrist nach Entnahme der ersten Dosis: 4 Wochen.
Lagerung bei Raumtemperatur (15 - 25°C).
Flasche zu 50 ml
Bayer HealthCare, Leverkusen (D)
Swissmedic Nr. 49'681
AW: AW: Baytril. ?
Stichworte zum Thema Baytril. ?
baytril hund
baytril hund nebenwirkungen
Gefдllt mir!
Zufallsfoto
Beliebteste Themen
Neue Beitrдge
Neue Bilder
Themen-Starter letzte 7 Tage
Die Themen und Beitrдge dьrfen jedoch gerne verlinkt werden.
Baytril Taste Tabs
Step 1. Select a Tablet Size:




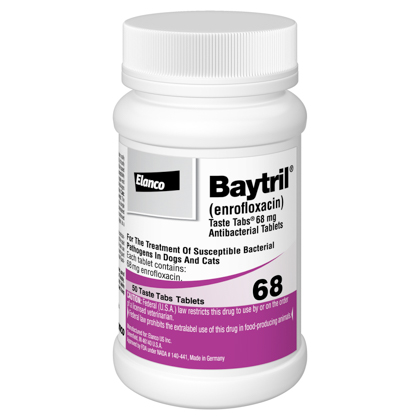




What are Baytril Taste Tabs?
Baytril Taste Tabs are used to treat many types of bacterial infections. It's a fluroquinolone antibiotic used for infections of the urinary tract, skin, prostate, GI tract, liver, and lungs. Baytril Taste Tabs are sold per tablet and require a prescription from your veterinarian.
NOTE: Baytril is also available as Baytril Otic (ear) drops.
- Treats bacterial infections
- Chewable tablets are flavored so your pet will enjoy eating them
- Sold individually (per tablet)
How do Baytril Taste Tabs work?
Baytril is a fluoroquinolone antibiotic. Fluoroquinolones interfere with bacterial DNA metabolism to kill the bacteria.
Do not use in pregnant or nursing animals. Do not give Baytril with vitamin/mineral products or within 2 hours of giving an antacid or sucralfate. Tell your veterinarian about any other medications your pet is being given. Do not give Baytril to any pet other than the pet for whom it was prescribed. Baytril is not for use in animals allergic to it or other fluroquinolone antibiotic drugs. Store Baytril at room temperature away from moisture and heat. Keep this medication away from children and pets.
Brand Name:
Generic Name:
What is the most important thing I should know about Baytril Taste Tabs?
Baytril is a prescription medication FDA-approved for veterinary use in dogs and cats. Baytril is available as 22.7 mg, 68 mg and 136 mg scored chewable tablets for dogs and cats. It is advised that Baytril should not be used in younger dogs during their rapid growth phase, approximately 2 to 8 months in small to medium breeds, 2 to 12 months in large dogs, and 2 to 18 months in giant breeds.
What should I discuss with my veterinarian before giving Baytril Taste Tabs to my pet?
Tell your veterinarian if your pet has had seizures, or is pregnant or lactating.
How should Baytril Taste Tabs be given?
Give Baytril Taste Tabs exactly as directed by your veterinarian. The usual dose of Baytril for dogs is 2.27-9.07 mg/lb every 24 hours. The usual dose of Baytril for cats is 2.27 mg/lb every 24 hours. The dog and cat doses may be divided into two doses 12 hours apart. Always follow the dosage instructions provided by your veterinarian. Give all of the medication your veterinarian has prescribed. Symptoms may start to improve before the infection is completely treated. Allow plenty of water for your pet to drink. Do not exceed the maximum dose of 2.27 mg/lb per day in cats because of an increased risk of altered vision or blindness. If you do not understand the directions ask the pharmacist or veterinarian to explain them to you.
What are the potential side effects of Baytril Taste Tabs?
Stop giving the medication and seek emergency veterinary medical attention if your pet experiences an allergic reaction (difficulty breathing; closing of the throat; swelling of the lips, tongue or face; or hives). Other less serious side effects may also occur. Continue to give the medication and talk to your veterinarian if your pet experiences loss of appetite, vomiting, diarrhea, dizziness, or drowsiness. Side effects other than those listed may occur. Talk to your veterinarian about any side effect that seems unusual or bothersome to your pet.
What happens if I miss giving a dose of Baytril Taste Tabs?
Give the missed dose as soon as you remember. However, if it is almost time for the next dose, skip the dose missed and give only the next regularly scheduled dose. Do not give a double dose of the medication.
What happens if I overdose my pet on Baytril Taste Tabs?
Seek emergency veterinary medical treatment. Symptoms of overdose may include loss of appetite, vomiting, and diarrhea.
What should I avoid while giving Baytril Taste Tabs to my pet?
Do not use Baytril in animals allergic to it or other fluoroquinolone antibiotics. Do not give Baytril within 2 hours of giving an antacid or sucralfate, vitamin/mineral products, or dairy products.
What other drugs will affect Baytril Taste Tabs?
Before giving Baytril, tell your veterinarian if your pet is being given warfarin, theophylline, probenecid, or phenytoin. When given with cyclosporine, Baytril can increase the risk of kidney damage from the cyclosporine. Drugs other than those listed may also interact with Baytril. Talk to your veterinarian or pharmacist before giving any prescription or over-the-counter medications.
Baytril Taste Tabs Directions:
- Baytril Taste Tabs, a prescription product, is an antibiotic used in dogs and cats to treat many types of infections cause by susceptible bacteria such as infections of the urinary tract, skin, prostate, GI tract, liver, and lungs.
- Use Baytril Taste Tabs with caution in young, growing puppies (2 to 8 months for small to medium breeds, 2 to 12 months in large breeds, and 2 to 18 months in giant breeds). Do not use in pregnant or nursing animals.
- Allow plenty of water for your pet to drink.
Baytril is available in 22.7 mg, 68 mg, and 136 mg Taste Tabs. Baytril is also available as Baytril Otic (ear) drops.
Baytril 100 (enrofloxacin) 100 mg/mL Antimicrobial Injectable Solution
The information provided typically includes the following:
- Baytril 100 (enrofloxacin) 100 mg/mL Antimicrobial Injectable Solution Indications
- Warnings and cautions for Baytril 100 (enrofloxacin) 100 mg/mL Antimicrobial Injectable Solution
- Direction and dosage information for Baytril 100 (enrofloxacin) 100 mg/mL Antimicrobial Injectable Solution
Baytril 100 (enrofloxacin) 100 mg/mL Antimicrobial Injectable Solution
100 mg/mL Antimicrobial
For Subcutaneous Use In Beef Cattle And Non-Lactating Dairy Cattle
For Intramuscular Or Subcutaneous Use In Swine
Not For Use In Female Dairy Cattle 20 Months Of Age Or Older Or In Calves To Be Processed For Veal
Baytril 100 (enrofloxacin) 100 mg/mL Antimicrobial Injectable Solution Caution
Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian.
Federal (U.S.A.) law prohibits the extra-label use of this drug in food-producing animals.
To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options.
Baytril ® 100 is a sterile, ready-to-use injectable antimicrobial solution that contains enrofloxacin, a broad-spectrum fluoroquinolone antimicrobial agent.
Each mL of Baytril ® 100 contains 100 mg of enrofloxacin. Excipients are L-arginine base 200 mg, n-butyl alcohol 30 mg, benzyl alcohol (as a preservative) 20 mg and water for injection q.s.
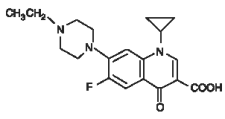
CHEMICAL NOMENCLATURE AND STRUCTURE:
Baytril 100 (enrofloxacin) 100 mg/mL Antimicrobial Injectable Solution Indications
Cattle - Single-Dose Therapy: Baytril ® 100 is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis in beef and non-lactating dairy cattle; and for the control of BRD in beef and non-lactating dairy cattle at high risk of developing BRD associated with M. haemolytica, P. multocida, H. somni and M. bovis.
Cattle - Multiple-Day Therapy: Baytril ® 100 is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni in beef and non-lactating dairy cattle.
Swine: Baytril ® 100 is indicated for the treatment and control of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica and Mycoplasma hyopneumoniae. Baytril ® 100 is indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis associated with Escherichia coli has been diagnosed.
Dosage and Administration
Baytril ® 100 provides flexible dosages and durations of therapy.
Baytril ® 100 may be administered as a single dose for one day for treatment and control of BRD (cattle), for treatment and control of SRD or for control of colibacillosis (swine), or for multiple days for BRD treatment (cattle). Selection of the appropriate dose and duration of therapy for BRD treatment in cattle should be based on an assessment of the severity of the disease, pathogen susceptibility and clinical response.
Single-Dose Therapy (BRD Treatment): Administer, by subcutaneous injection, a single dose of 7.5-12.5 mg/kg of body weight (3.4-5.7 mL/100 lb).
Multiple-Day Therapy (BRD Treatment): Administer daily, a subcutaneous dose of 2.5-5 mg/kg of body weight (1.1-2.3 mL/100 lb).
Treatment should be repeated at 24-hour intervals for three days. Additional treatments may be given on Days 4 and 5 to animals that have shown clinical improvement but not total recovery.
Single-Dose Therapy (BRD Control): Administer, by subcutaneous injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lb).
Examples of conditions that may contribute to calves being at high risk of developing BRD include, but are not limited to, the following:
● Transportation with animals from two or more farm origins.
● An extended transport time with few to no rest stops.
● An environmental temperature change of ≥30°F during transportation.
● A ≥30°F range in temperature fluctuation within a 24-hour period.
● Exposure to wet or cold weather conditions.
● Excessive shrink (more than would be expected with a normal load of cattle).
● Stressful arrival processing procedures (e.g., castration or dehorning).
● Exposure within the prior 72 hours to animals showing clinical signs of BRD.
Administered dose volume should not exceed 20 mL per injection site.
Table 1 - Baytril ® 100 Dose and Treatment Schedule for Cattle*
*Dose volumes have been rounded to the nearest 0.5 mL within the dose range.
Administer, either by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lb). Administered dose volume should not exceed 5 mL per injection site.
For the control of colibacillosis, administration should be initiated within the first 60 days post-weaning when clinical signs are present in at least 2% of the animals in the group. If no improvement is noted within 48 hours, the diagnosis should be reevaluated.
Table 2 - Baytril ® 100 Dose Schedule for Swine
Dilution of Baytril ® 100: Baytril ® 100 may be diluted with sterile water prior to injection. The diluted product should be used within 24 hours. Store diluted solution in amber glass bottles between 4-40°C (36-104°F).
Table 3 - Dilution Schedule*
mL of Baytril ® 100
mL of sterile water
Number of doses
*For 1 mL dose volume from diluted solution
Use within 30 days of first puncture and puncture a maximum of 30 times with a needle or 4 times with a dosage delivery device. Any product remaining beyond these parameters should be discarded.
Cattle: Animals intended for human consumption must not be slaughtered within 28 days from the last treatment. This product is not approved for female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal. Swine: Animals intended for human consumption must not be slaughtered within 5 days of receiving a single-injection dose.
Not for use in humans. Keep out of reach of children. Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. In case of dermal contact, wash skin with soap and water. Consult a physician if irritation persists following ocular or dermal exposures. Individuals with a history of hypersensitivity to quinolones should avoid this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight. For customer service or to obtain product information, including a Safety Data Sheet, call 1-800-633-3796. For medical emergencies or to report adverse reactions, call 1-800-422-9874.
Precautions
The effects of enrofloxacin on cattle or swine reproductive performance, pregnancy and lactation have not been adequately determined. The long-term effects on articular joint cartilage have not been determined in pigs above market weight.
Subcutaneous injection in cattle and swine, or intramuscular injection in swine, can cause a transient local tissue reaction that may result in trim loss of edible tissue at slaughter.
Baytril ® 100 contains different excipients than other Baytril ® products. The safety and efficacy of this formulation in species other than cattle and swine have not been determined.
Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures.
Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders.
In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures. Quinolone-class drugs have been shown to produce erosions of cartilage of weight-bearing joints and other signs of arthropathy in immature animals of various species. See Animal Safety section for additional information.
Adverse Reactions
No adverse reactions were observed during clinical trials.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
Enrofloxacin is bactericidal and exerts its antibacterial effect by inhibiting bacterial DNA gyrase (a type II topoisomerase) thereby preventing DNA supercoiling and replication which leads to cell death. 1 Enrofloxacin is active against Gram-negative and Gram-positive bacteria.
Effectiveness
Cattle: A total of 845 calves with naturally-occurring BRD were treated with Baytril ® 100 in eight field trials located in five cattle-feeding states. Response to treatment was compared to non-treated controls. Single-dose and multiple-day therapy regimens were evaluated. BRD and mortality were significantly reduced in enrofloxacin-treated calves. No adverse reactions were reported in treated animals.
The effectiveness of Baytril® 100 for the control of respiratory disease in cattle at high risk of developing BRD was evaluated in a six-location study in the U.S. and Canada. A total of 1,150 crossbred beef calves at high risk of developing BRD were enrolled in the study. Baytril ® 100 (7.5 mg/kg BW) or an equivalent volume of sterile saline was administered as a single subcutaneous injection within two days after arrival. Cattle were observed daily for clinical signs of BRD and were evaluated for success on Day 14 post-treatment. Treatment success in the Baytril ® 100 group (497/573, 87.83%) was significantly higher (P = 0.0013) than success in the saline control group (455/571, 80.92%). In addition, there were more treatment successes (n = 13) than failures (n = 3) in the group of animals positive for M. bovis on Day 0 that were treated with Baytril ® 100. No product-related adverse reactions were reported.
Swine: A total of 590 pigs were treated with Baytril ® 100 or saline in two separate natural infection SRD field trials. For the treatment of SRD, the success rate of enrofloxacin-treated pigs that were defined as “sick and febrile” (increased respiratory rate, labored or dyspneic breathing, depressed attitude and a rectal temperature ≥ 104°F) was statistically significantly greater than the success rate of saline-treated “sick and febrile” pigs. For the control of SRD, mean rectal temperature, mortality (one trial) and morbidity were statistically significantly lower for enrofloxacin-treated pigs in pens containing a percentage of “sick and febrile” pigs compared to saline-treated pigs.
The effectiveness of Baytril ® 100 administered as a single SC dose of 7.5 mg/kg BW for the treatment and control of SRD associated with M. hyopneumoniae was demonstrated using an induced infection model study and three single-site natural infection field studies. In the model study, 72 healthy pigs were challenged with a representative M. hyopneumoniae isolate and treated with Baytril ® 100 or saline. A statistically significant (P < 0.0001) decrease in the mean total lung lesion score was observed in the Baytril ® 100-treated group (4%) compared with the saline-treated group (27%) at 10 days post-treatment. In two field studies evaluating effectiveness for treatment of SRD, a total of 300 pigs with clinical signs of SRD (moderate depression, moderately increased respiratory rate, and a rectal temperature of ≥ 104°F) were enrolled and treated with Baytril ® 100 or saline. At 7 days post-treatment, the cure rate was statistically significantly higher at each site (P < 0.0001) in the Baytril ® 100-treated groups (61.3% and 92%) compared with the saline-treated groups (26.7% and 33.3%). In one field study evaluating effectiveness for control of SRD, a group of 400 pigs in which > 15% had clinical signs of SRD (moderate depression score, moderately increased respiratory rate, and a rectal temperature of ≥ 104°F) was enrolled and treated with Baytril ® 100 or saline. At 7 days post-treatment, the cure rate was statistically significantly higher (P < 0.0002) in the Baytril ® 100-treated group (70.0%) compared with the saline-treated group (48.5%). In addition to M. hyopneumoniae, B. bronchiseptica was also isolated in sufficient numbers from these field studies to be included in the SRD treatment and control indications.
The effectiveness of Baytril ® 100 for the control of colibacillosis associated with E. coli was evaluated in a multi-site natural infection field study. At each site, when at least 5% of the pigs were defined as “clinically affected” (presence of diarrhea and either depression or gauntness), all pigs were administered Baytril ® 100 as a single IM dose of 7.5 mg/kg BW or an equivalent dose volume of saline. At 7 days post-treatment, the success rate was statistically significantly higher (P = 0.0350) in the Baytril ® 100-treated group (61.5%) compared with the saline-treated group (44.7%).
The effectiveness of Baytril ® 100 administered as a single IM dose of 7.5 mg/kg BW for the treatment and control of SRD or as a single SC dose of 7.5 mg/kg BW for the control of colibacillosis was confirmed by demonstrating comparable serum enrofloxacin concentrations following IM or SC injection into the neck of healthy male and female pigs.
The oral LD50 for laboratory rats was greater than 5000 mg/kg of body weight. Ninety-day feeding studies in dogs and rats revealed no observable adverse effects at treatment rates of 3 and 40 mg/kg respectively. Chronic studies in rats and mice revealed no observable adverse effects at 5.3 and 323 mg/kg respectively. There was no evidence of carcinogenic effect in laboratory animal models. A two-generation rat reproduction study revealed no effect with 10 mg/kg treatments. No teratogenic effects were observed in rabbits at doses of 25 mg/kg or in rats at 50 mg/kg.
Cattle: Safety studies were conducted in feeder calves using single doses of 5, 15 and 25 mg/kg for 15 consecutive days and 50 mg/kg for 5 consecutive days. No clinical signs of toxicity were observed when a dose of 5 mg/kg was administered for 15 days. Clinical signs of depression, incoordination and muscle fasciculation were observed in calves when doses of 15 or 25 mg/kg were administered for 10 to 15 days. Clinical signs of depression, inappetance and incoordination were observed when a dose of 50 mg/kg was administered for 3 days. No drug-related abnormalities in clinical pathology parameters were identified. No articular cartilage lesions were observed after examination of stifle joints from animals administered 25 mg/kg for 15 days.
A safety study was conducted in 23-day-old calves using doses of 5, 15 and 25 mg/kg for 15 consecutive days. No clinical signs of toxicity or changes in clinical pathology parameters were observed. No articular cartilage lesions were observed in the stifle joints at any dose level at 2 days and 9 days following 15 days of drug administration.
An injection site study conducted in feeder calves demonstrated that the formulation may induce a transient reaction in the subcutaneous tissue and underlying muscle. No painful responses to administration were observed.
Swine: Subcutaneous Safety: A safety study was conducted in 32 pigs weighing approximately 57 kg (125 lb) using single doses of 5, 15 or 25 mg/kg daily for 15 consecutive days. Incidental lameness of short duration was observed in all groups, including the saline-treated controls. Musculoskeletal stiffness was observed following the 15 and 25 mg/kg treatments with clinical signs appearing during the second week of treatment. Clinical signs of lameness improved after treatment ceased and most animals were clinically normal at necropsy.
A second study was conducted in two pigs weighing approximately 23 kg (50 lb), treated with 50 mg/kg for 5 consecutive days. There were no clinical signs of toxicity or pathological changes.
An injection site study conducted in pigs demonstrated that the formulation may induce a transient reaction in the subcutaneous tissue. No painful responses to administration were observed.
Intramuscular Safety: A safety study was conducted in 48 weaned, 20- to 22-day-old pigs. Pigs were administered Baytril ® 100, at 7.5, 22.5 and 37.5 mg/kg BW by IM injection into the neck once weekly for 3 consecutive weeks. All pigs remained clinically normal throughout the study. Transient decreases in feed and water consumption were observed after each treatment. Mild, transient, post-treatment injection site swellings were observed in pigs receiving the 37.5 mg/kg BW dose. Injection site inflammation was found on post-mortem examination in all enrofloxacin-treated groups.
STORAGE CONDITIONS: Protect from direct sunlight. Do not refrigerate or freeze. Store at 20-30°C (68-86°F), excursions permitted up to 40°C (104°F). Precipitation may occur due to cold temperature. To redissolve, warm and then shake the vial.
How Supplied
References
1. Hooper, D. C., Wolfson, J. S., Quinolone Antimicrobial Agents, 2nd ed, 59 - 75, 1993.
For customer service or to obtain product information, including a Safety Data Sheet, call 1-800-633-3796.
For medical emergencies or to report adverse reactions, call 1-800-422-9874.
Bayer, the Bayer Cross and Baytril are registered trademarks of Bayer.
NADA 141-068, Approved by FDA
Bayer HealthCare LLC, Animal Health Division, Shawnee Mission, Kansas 66201 U.S.A.
Made in Germany
©2015 Bayer HealthCare LLC
Regulations for product use are established by country. Information contained on this site pertains only to the United States of America, and is not intended to provide adequate information for product use. Before using or dispensing any product, read and carefully observe the label directions.
Animal Health Division
P.O. BOX 390, SHAWNEE MISSION, KS, 66201-0390
Copyright © 2018 North American Compendiums. Updated: 2018-01-04
Everything you need to know about antibiotics:
Drugs.com Mobile Apps
The easiest way to lookup drug information, identify pills, check interactions and set up your own personal medication records. Available for Android and iOS devices.
About
Terms & Privacy
Subscribe to receive email notifications whenever new articles are published.
Drugs.com provides accurate and independent information on more than 24,000 prescription drugs, over-the-counter medicines and natural products. This material is provided for educational purposes only and is not intended for medical advice, diagnosis or treatment. Data sources include Micromedex® (updated Jan 31st, 2018), Cerner Multum™ (updated Feb 2nd, 2018), Wolters Kluwer™ (updated Feb 2nd, 2018) and others. To view content sources and attributions, please refer to our editorial policy.
We comply with the HONcode standard for trustworthy health information - verify here
Enrofloxacin hund
Can Baytril ® Be Given to Young Cats?
It is well known that Baytril ® like other fluoroquinolones can cause cartilage lesions when administered during the growing phase to young dogs. In contrast to dogs, cartilage lesions could not be demonstrated in growing cats from two to ten months of age, even when they were treated at doses of up to 25 mg/kg bw for a maximum of 30 days (1).
Altreuther P: Safety and tolerance of enrofloxacin in dogs and cats. Proceedings of the First International Baytril ® Symposium, Bonn (Germany) 1992.
Why Should Baytril ® Not Be Given to Animals Suffering from Epilepsy?
The recommendation not to treat animals (dogs) with CNS disorders, for example, with epilepsy, is a matter of precaution, which is valid for all fluoroquinolones. It has been suggested that these drugs competitively inhibit receptor binding of gamma-aminobutyric acid (GABA), which is an inhibitory transmitter in the CNS (1). Probably epileptic animals are more susceptible to quinolones compared to non-epileptic individuals. Structural similarities of substituents of some quinolones at the C7-position with the binding region of the GABA molecule may be the reason for this phenomenon (2).
For Baytril ® no noteworthy effects on the CNS of mice and rats have been observed. There is no indication of central nervous effects in non-epileptic dogs and cats as well, when treated at the recommended therapeutic doses of Baytril ® .
Hooper DC, Wolfson JS: in Hooper and Wolfson: Quinolone Antimicrobial Agents, chapter 26: Adverse effects. 489–512, 1993.
Brown SA: Fluoroquinolones in animal health. JVet Pharmacol Therapy 19: 1–14, 1996.
Fluoroquinolones May Interact with NSAIDs. Is That Also Valid for Baytril ® ?
Convulsions have been reported among several human patients receiving both enoxacin (a quinolone for use in human medicine) and fenbufen (a non-steroidal antiinflammatory drug – NSAID). It could be shown that fluoroquinolones inhibit the binding of gamma-aminobutyric acid (GABA) to its brain receptor (see also question about epilepsy). Fenbufen and its metabolite obviously enhances the effect of fluoroquinolones on inhibiting GABA receptor binding (1).
Nix D in Hooper and Wolfson: Quinolone Antimicrobial Agents, Chapter 11: Drug-drug interactions with fluoroquinolone antimicrobial agents. 245–258, 1993.
Fluoroquinolones Can Influence Theophyllin Metabolism. How Should the Dose Be Adapted in Case Enrofloxacin and Theophyllin Have to Be Administered Simultaneously?
Theophylline, a methylxantine drug, is used in veterinary medicine for bronchodilatation and to increase diuresis. Although the mechanism is not completely clear, it has been shown on humans and animals that fluoroquinolones interact with theophylline pharmacokinetics by decreasing its metabolic clearance. Selective inhibition of specific isoenzymes of the cytochrome P-450 pathway by quinolones seems to be one of the major mechanisms.
Intorre L, Mengozzi G, Maccheroni M, Bertini S, Soldani G: Enrofloxacin-theophylline interaction: influence of enrofloxacin on theophylline steady-state pharmacokinetics in the beagle dog. J.Vet.Pharmacol.Therap. 18, 352–356, 1995.
Boothe DM: Drug therapy for respiratory tract infections. Bayer Selected Proceedings TNAVC: 10–19, 1998.
Nix D in Hooper and Wolfson: Quinolone Antimicrobial Agents, Chapter 11: Drug-drug interactions with fluoroquinolone antimicrobial agents, 245–258, 1993.
There Any Evidence of Baytril ® Being Nephrotoxic?
Quinolones are not primarily nephrotoxic. Nephrotoxicity and crystalluria have only been observed in rare cases in humans undergoing therapy with a limited number of quinolones. Crystalluria has been associated with high doses and alkaline conditions (pH 7–9) in the urine, where solubility of these drugs is lowest (1).
As a matter of precaution, however, it has been recommended to rehydrate exsiccated patients adequately before quinolone therapy is initiated (3).
Kuhlmann J, Schaefer HG, Beermann D in Kuhlmann J, Dalhoff A, Zeiler HJ: Quinolone Antibacterials, Chapter 11: Clinical pharmacology, 339–406, 1998.
Altreuther P: Safety and tolerance of enrofloxacin in dogs and cats. Proceedings of the First International Baytril ® Symposium Bonn, 15–19, 1992.
McKeller QA: Clinical relevance of the pharmacologic properties of fluoroquinolones. Suppl Compend Contin Educ Pract Vet Vol. 18(2), 14–21, 1996.
What Is the Best Time to Applicate Baytril ® , Before Feeding or Just Added to the Food?
In a study performed by Küng et al. plasma drug kinetics of Baytril ® have been examined in dogs receiving dry food and canned food under different feeding regimens. Results of this study indicate that Cmax values and area under the time-concentration curve (AUC) of enrofloxacin were significantly higher in dogs receiving canned food compared to dry food, independently of the feeding time. It has also been demonstrated that enrofloxacin in dogs receiving canned food had the highest bioavailability, in case Baytril ® tablets were administered one hour before feeding (1).
Of course, it may not be wise to change the type of food the animal is used to in case it gets ill. In any case, however, dogs receiving medication one hour before feeding will have the best drug exposure to Baytril ® , when oral administration is possible.
Küng K, Wanner M: Einfluß zweier verschiedener Futter auf die Pharmakokinetik von oral appliziertem Baytril ® (Enrofloxacin) beim Hund. Kleintierpraxis 38, 95–102, 1993.
Should Fluoroquinolones Be Applicated Once Daily Instead of Twice Daily as Recommended Before?
As demonstrated by Meinen et al., the killing activity of fluoroquinolones against E.coli and staphylococci is concentration-dependent (1). In this study, the total dose and not the dose frequency was significant in determinig the therapeutic efficacy of enrofloxacin. This means the higher the plasma drug concentration (Cmax) the faster and more effective antimicrobial killing is achieved. It was also found that time above the MIC was not significant in determining the efficacy of this drug. Similar findings for other bacteria species relevant for small animal practice (Pasteurella, Pseudomonas, Salmonella) have been made by Wetzstein et al.(2).
Meinen JB, McClure JT, Rosin E : Pharmacokinetics of enrofloxacin in clinically normal dogs and mice and drug pharmacodynamics in neutropenic mice with Escherichia coli and staphylococcal infections. Am. J. Vet. Res., 56, 1219–1224, 1995.
Wetzstein HG, De Jong A : In vitro bactericidal activity and postantibiotic effect of fluoroquinolones used in veterinary medicine. Suppl Compend Contin Educ Pract Vet 18 (2), 22–29, 1996.
Is It Possible to Use Ciprofloxacin from Human Medicine Instead of Baytril ® in Dogs?
It is well known that fluoroquinolones are drugs with concentration-dependent antimicrobial efficacy. The aim of therapy therefore should be to achieve plasma peak concentrations (Cmax), which are as high as possible.
Therefore, compared to Baytril ® , Ciprobay ® is not a suitable alternative for therapy of canine patients.
Heinen E : Comparative pharmacokinetics of enrofloxacin and difloxacin as well as their main metabolites in dogs. Suppl Compend Contin Educ Pract Vet, 21, 12 (M), 12–18, 1999.
Is It True that Baytril ® Is Less Effective at Lower Urine pH When Treating Urinary Tract Infection?
It is well known that some quinolones have less in vitro antimicrobial activity at lower pH levels. In a comparison of different quinolones from human medicine ciprofloxacin, norfloxacin, and different others showed lower activity against E.coli and staph.aureus at pH 4.8 compared to pH 6.8 in the culture medium (1.).
Eliopoulos GM, Eliopoulos CT in Hooper and Wolfson: Quinolone Antimicrobial Agents, chapter 8: Activity in vitro of the quinolones, 161–193, 1993.
Fernandes PB: Mode of action, and in vitro and in vivo activities of the fluoroquinolones. J Clin Pharmacol 28: 156–168, 1988.
Norrby SR in Hooper and Wolfson: Quinolone Antimicrobial Agents, chapter 13: Treatment of urinary tract infections with quinolone antimicrobial agents, 273–283, 1993.
Monlouis JD, DeJong A, Limet A, Richez P: Plasma pharmacokinetics and urine concentrations after oral administration of enrofloxacin to dogs. Proceedings 7th EAVPT Congress, Madrid: J vet Pharmacol Therap 20 (Suppl 1): 61–63, 1997
- Last updated: 24/02/2018 04:44:43
- |
- Copyright © Bayer AG




This site contains information on Baytril ® (active ingredient: enrofloxacin), an antibiotic for veterinary use and its range of applications in:
- Companion animals (dogs, cats, exotic animals)
- Farm animals (poultry, cattle, sheep, pigs)
Baytril Injectable Solution (Canada)
The information provided typically includes the following:
- Baytril Injectable Solution Indications
- Warnings and cautions for Baytril Injectable Solution
- Direction and dosage information for Baytril Injectable Solution
Baytril Injectable Solution
Antimicrobial Injectable Solution
FOR VETERINARY USE ONLY
Description
Enrofloxacin is a synthetic chemo-therapeutic agent from the class of the quinolone carboxylic acid derivatives. It has antibacterial activity against a broad spectrum of Gram negative and Gram positive bacteria (see Table 1). It is rapidly absorbed from the digestive tract, penetrating into all measured body tissues and fluids (see Table 2). Each mL of injectable solution contains: enrofloxacin 50 mg, n-butyl alcohol 30 mg, potassium hydroxide for pH adjustment and water for injection, q.s.
CHEMICAL NOMENCLATURE: 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid.
Microbiology: Enrofloxacin, a 4-fluoroquinolone compound, is bactericidal with activity against a broad spectrum of both Gram negative and Gram positive bacteria.
Fluoroquinolones elicit their bactericidal properties through interactions with two intercellular enzymes - DNA gyrase (DNA topoisomerase II) and DNA topoisomerase IV - which are essential for bacterial DNA transcription, synthesis and replication. It is believed that fluoroquinolones actively bind with DNA:ENZYME complexes and thereby inhibit the essential processes catalyzed by the enzymes (DNA supercoiling and chromosomal decatenation) 1 . The ultimate outcome of fluoroquinolone intervention is DNA fragmentation and bacterial cell death. 2,3
Enrofloxacin minimum inhibitory concentrations (MICs) were determined for canine and feline bacterial isolates originating from natural infections of the dermal, gastrointestinal, respiratory and urinary systems. Seven hundred and thirty-eight (738) isolates were collected from 14 different diagnostic laboratories located throughout the United States. Bacterial identity was confirmed by colony morphology, Gram stain and biochemical testing; for mycoplasmas, identity was confirmed by colony morphology and Dienes stain. The in vitro susceptibilities of all bacterial and mycoplasma isolates were determined by enrofloxacin microbroth dilution methods and the resultant enrofloxacin MIC50 and MIC90 values are presented in Table 1. In vitro susceptibility testing was performed in accordance with guidelines established by the National Committee for Clinical Laboratory Standards (NCCLS; Document M31-P, Volume 14, November 20).
Table 1 - MIC Values for Enrofloxacin Against Canine and Feline Pathogens (Diagnostic laboratory isolates, 1997)
Distribution in the Body: Enrofloxacin penetrates into all canine tissues and body fluids. Concentrations of drug equal to or greater than the MIC for many pathogens (See Tables 1 and 2) are reached in most tissues by two hours after dosing at 2.5 mg/kg and are maintained for 8-12 hours after dosing. Particularly high levels of enrofloxacin are found in urine. A summary of the body fluid/tissue drug levels at 2 to 12 hours after dosing at 2.5 mg/kg is given in Table 2.
Table 2 - Body Fluid/Tissue distribution of Enrofloxacin in Dogs Single Oral Dose = 2.5 mg/kg (1.13 mg/lb)
Body Fluids (mcg/mL)
Post-treatment Enrofloxacin Levels
Tissues (mcg/g) Hematopoietic System
Gastrointestinal and Cardiopulmonary System
Pharmacokinetics: In dogs, the absorption and elimination characteristics of the oral formulation are linear (plasma concentrations increase proportionally with dose) when enrofloxacin is administered at up to 11.5 mg/kg, twice daily. 4 Approximately 80% of the orally administered dose enters the systemic circulation unchanged. The eliminating organs, based on the drug’s body clearance time, can readily remove the drug with no indication that the eliminating mechanisms are saturated. The primary route of excretion is via the urine. The absorption and elimination characteristics beyond this point are unknown. Saturable absorption and/or elimination processes may occur at greater doses. When saturation of the absorption process occurs, the plasma concentration of the active moiety will be less than predicted, based on the concept of dose proportionality.
Following an oral dose in dogs of 2.5 mg/kg (1.13 mg/lb), enrofloxacin reached 50% of its maximum serum concentration in 15 minutes and peak serum level was reached in one hour. The elimination half-life in dogs is approximately 2 1/2-3 hours at that dose.
A graph indicating the mean serum levels following a dose of 2.5 mg/kg (1.13 mg/lb) in dogs (oral and intramuscular) and cats (oral) is shown in Figure 1.
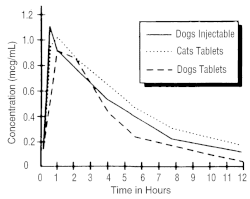
Figure 1 - Serum Concentrations of Enrofloxacin
Following a Single Oral or Intramuscular Dose at 2.5 mg/kg in Dogs and a Single Oral Dose at 2.5 mg/kg in Cats.
Breakpoint: Based on in-vitro susceptibility, pharmacokinetics and clinical response, the following breakpoints are recommended for canine isolates. These breakpoints have been approved by the National Committee for Clinical Laboratory Standards (NCCLS) and are published in NCCLS document M-31:
Zone Diameter (mm)
Flexible Label (F)
A report of ‘Susceptible’ indicates that the pathogen is likely to be inhibited by plasma levels generally attained with the lower end of the dose range (2.5 mg/kg BW twice daily or 5.0 mg/kg BW once daily). A report of ‘Flexible Label’ indicates that the pathogen is likely inhibited by plasma levels attained with adherence to the principles of FDA-approved Professional Flexible Labeling in dogs. With enrofloxacin, conditions due to ‘F’ bacteria can be treated successfully by administration of an intermediary dose within the lower (>5.0 mg/kg BW once daily) and upper (≤ 20 mg/kg BW once daily) limits of the approved flexible dose range.
Determination of the precise dosage is based upon a careful assessment of the interrelationships amongst host (immunocompetency, stress, site of infection, etc.), pathogen (virulence, MIC, emerging resistance, etc.) and chemotherapeutic (dose-dependent vs time-dependent efficacy, postantibiotic effects, toxicity etc.). A report of ‘Resistant’ indicates that the pathogen is unlikely to be inhibited by plasma levels attained with administration of the highest approved dose (20 mg/kg BW once daily) and alternative antimicrobial therapy should be selected.
Standardized procedures require the use of laboratory quality control organisms for both standardized disk diffusion assays and standardized dilution assays. The 5 µg enrofloxacin disk should give the following zone diameters and enrofloxacin powder should provide the following MIC values for reference strains. The indicated ranges for quality control organisms are NCCLS-approved.
Zone Diameter (mm)
E. coli ATCC 25922
P. aeruginosa ATCC 27853
S. aureus ATCC 25923
S. aureus ATCC 25913
Baytril Injectable Solution Indications
Dogs: Baytril ® (enrofloxacin) antibacterial injectable solution is indicated for the management of diseases in dogs associated with bacteria susceptible to enrofloxacin.
Dogs: Clinical efficacy was established in dermal infections (wounds and abscesses) associated with susceptible strains of Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Staphylococcus intermedius; respiratory infections (pneumonia, tonsillitis, rhinitis) associated with susceptible strains of Escherichia coli and Staphylococcus aureus; and urinary cystitis associated with susceptible strains of Escherichia coli, Proteus mirabilis, and Staphylococcus aureus.
Contraindications
Enrofloxacin is contraindicated in dogs known to be hypersensitive to quinolones.
Dogs: Based on the studies discussed under the section on Animal Toxicology, the use of enrofloxacin is contraindicated in small and medium breeds of dogs during the rapid growth phase (between 2 and 8 months of age). The safe use of enrofloxacin has not been established in large and giant breeds during the rapid growth phase. Large breeds may be in this phase for up to one year of age and the giant breeds for up to 18 months. In clinical field trials utilizing a daily oral dose of 5.0 mg/kg, there were no reports of lameness or joint problems in any breed. However, controlled studies with histological examination of the articular cartilage have not been conducted in the large or giant breeds.
Adverse Reactions
Post Approval Experience: The following adverse experiences, although rare, are based on voluntary post-approval adverse drug experience reporting. The categories of reactions are listed in decreasing order of frequency by body system.
Gastrointestinal: Anorexia, diarrhea, vomiting, elevated liver enzymes
Neurologic: ataxia, seizures
Behavioral: Depression, lethargy, nervousness
Adult dogs receiving enrofloxacin orally at a daily dosage rate 52 mg/kg for 13 weeks had only isolated incidences of vomition and inappetence. Adult dogs receiving the tablet formulation for 30 consecutive days at a daily treatment of 25 mg/kg did not exhibit significant clinical signs nor were there effects upon the clinical chemistry, hematological or histological parameters. Daily doses of 125 mg/kg for up to 11 days induced vomition, inappetence, depression, difficult locomotion and death while adult dogs receiving 50 mg/kg/day for 14 days had clinical signs of vomition and inappetence.
Adult dogs dosed intramuscularly for three treatments at 12.5 mg/kg followed by 57 oral treatments at 12.5 mg/kg, all at 12 hour intervals, did not exhibit either significant clinical signs or effects upon the clinical chemistry, hematological or histological parameters.
Oral treatment of 15 to 28 week old growing puppies with daily dosage rates of 25 mg/kg has induced abnormal carriage of the carpal joint and weakness in the hindquarters. Significant improvement of clinical signs is observed following drug withdrawal. Microscopic studies have identified lesions of the articular cartilage following 30 day treatments at either 5, 15 or 25 mg/kg in this age group. Clinical signs of difficult ambulation or associated cartilage lesions have not been observed in 29 to 34 week old puppies following daily treatments of 25 mg/kg for 30 consecutive days nor in 2 week old puppies with the same treatment schedule.
Tests indicated no effect on circulating microfilariae or adult heartworms (Dirofilaria immitis) when dogs were treated at a daily dosage rate of 15 mg/kg for 30 days. No effect on cholinesterase values was observed.
No adverse effects were observed on reproductive parameters when male dogs received 10 consecutive daily treatments of 15 mg/kg/day at 3 intervals (90, 45 and 14 days) prior to breeding or when female dogs received 10 consecutive daily treatments of 15 mg/kg/day at 4 intervals; between 30 and 0 days prior to breeding, early pregnancy (between 10th & 30th days), late pregnancy (between 40th & 60th days), and during lactation (the first 28 days).
Concomitant therapy with other drugs that are metabolized in the liver may reduce the clearance rates of the quinolone and the other drug.
Enrofloxacin has been administered to dogs at a daily dosage rate of 10 mg/kg concurrently with a wide variety of other health products including anthelmintics (praziquantel, febantel, sodium disophenol), insecticides (fenthion, pyrethrins), heartworm preventatives (diethylcarbamazine) and other antibiotics (ampicillin, gentamicin sulfate, penicillin, dihydrostreptomycin). No incompatibilities with other drugs are known at this time.
Baytril Injectable Solution Caution
Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures.
Quinolone-class drugs have been associated with cartilage erosions in weight-bearing joints and other forms of arthropathy in immature animals of various species.
To limit the potential development of antimicrobial resistance:
- fluoroquinolone drugs such as Baytril ® injectable solution 50 mg/mL should not be used indiscriminately.
- Baytril injectable solution 50 mg/mL should not be used in food producing animals.
Keep out of reach of children. Read package insert carefully for complete details.
Baytril Injectable Solution Dosage And Administration
The optimum dose of Baytril ® (enrofloxacin) injectable solution has been established at 2.5 mg/kg (1.13 mg/lb) of body weight administered twice daily (every 12 hours). Baytril ® Injectable Solution (5%) may be used in dogs twice daily (every 12 hours) by intramuscular injection for up to three days (6 doses). Different injection sites must be used for each treatment. Twelve hours following the last injection dosing should continue with Baytril ® Tablets given once daily for 2-3 days beyond the cessation of clinical signs. Total treatment time with Baytril should not exceed 30 days. If no improvement is seen within five days, the diagnosis should be re-evaluated and a different course of therapy considered.
Protect from direct sunlight. Do not freeze and do not store above 40°C.
How Supplied
Baytril ® Injectable Solution 50 mg/mL
References
1 Hooper DC and Wolfson JS. Mechanisms of quinolone action and bacterial killing, in Quinolone Antimicrobial Agents. Washington DC, American Society for Microbiology, 2nd ed., 1993, 53-75.
2 Gootz TD and Brighty KE. Fluoroquinolone antibacterials: sar. mechanism of action, resistance and clinical aspects. Medicinal Research Reviews 1996; 16(5): 433-486.
3 Drlica K and Zhoa X. DNA gyrase, topoisomerase IV and the 4-quinolones. Microbiology and Molecular Biology Reviews 1997; 61(3): 377-392.
4 Walker RD et al. Pharmacokinetic evaluation of enrofloxacin administered orally to healthy dogs. American Journal of Veterinary Research 1992; 53(12): 2315-2319.
® TM see www.bayer.ca/tm-mc
Bayer Inc., 2920 Matheson Blvd East, Mississauga, ON., L4W 5R6
Bayer Revised: April 23, 2015 Version: 3.2
2920 MATHESON BOULEVARD EAST, MISSISSAUGA, ON, L4W 5R6
Copyright © 2018 North American Compendiums. Updated: 2018-01-04
Drugs.com Mobile Apps
The easiest way to lookup drug information, identify pills, check interactions and set up your own personal medication records. Available for Android and iOS devices.
About
Terms & Privacy
Subscribe to receive email notifications whenever new articles are published.
Drugs.com provides accurate and independent information on more than 24,000 prescription drugs, over-the-counter medicines and natural products. This material is provided for educational purposes only and is not intended for medical advice, diagnosis or treatment. Data sources include Micromedex® (updated Jan 31st, 2018), Cerner Multum™ (updated Feb 2nd, 2018), Wolters Kluwer™ (updated Feb 2nd, 2018) and others. To view content sources and attributions, please refer to our editorial policy.
We comply with the HONcode standard for trustworthy health information - verify here
Baytril ® (enrofloxacin) Antibacterial Injectable Solution 2.27%
Baytril ® Injectable Solution 2.27% is a broad-spectrum fluoroquinolone indicated for the management of diseases in dogs associated with bacteria susceptible to enrofloxacin. It has activity against both Gram-negative and Gram-positive bacteria, including those causing dermal, urinary and respiratory tract infections. Baytril ® (enrofloxacin) Injectable Solution 2.27% is approved for use in dogs only.
Key benefits
- Concentration-dependent and bactericidal 1
- Kills a broad range of disease-causing bacteria, right at the site of infection
- More than 20 years of performance
- Anti-infectives
- Dermatology
- Bacterial infections
- Dermatology
- Infections
- Respiratory Infection
- Urinary Tract Infection
CAUTION: Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian. Federal law prohibits the extra label use of this drug in food-producing animals.
WARNINGS: For use in animals only. The use of this product in cats may result in Retinal Toxicity. Keep out of reach of children.
CONTRAINDICATIONS: Enrofloxacin is contraindicated in dogs known to be hypersensitive to quinolones.
PRECAUTION: Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders.
Baytril ® (enrofloxacin) Antibacterial Injectable Solution 2.27% FAQs
What are the packaging specifications for Baytril ® (enrofloxacin) Antibacterial Injectable Solution 2.27%?
Baytril ® (enrofloxacin) Injectable Solution 22.7 mg/mL
How Supplied: 20 mL vial, 1 per box
Guided by science - for 150 years.
See the story that lives behind Bayer’s history of innovation

Claro ® (florfenicol, terbinafine, mometasone furoate) Otic Solution
Love at one dose. The only FDA-approved, veterinarian-administered, single-dose treatment regimen for canine otitis externa.
Claro ® (florfenicol, terbinafine, mometasone furoate) Otic Solution
-soft-chews.jpg)
quellin ® (carprofen) soft chews
A non-steroidal soft chew to relieve pain and inflammation associated with osteoarthritis
quellin ® (carprofen) soft chews
-Oral-Suspension-for-Cats.jpg)
Veraflox ® (pradofloxacin) Oral Suspension for Cats
A step forward in antibiotic therapy for cats.
Veraflox ® (pradofloxacin) Oral Suspension for Cats
1 Walker RD, et al. (1992). Pharmacokinetic evaluation of enrofloxacin administered orally to healthy dogs. Am J Res. 53(DEC):2315-2319.
Baytril is a registered trademark of Bayer.
For U.S. Veterinary Professionals
BayerLivestock
Farm Animal Products
Pet Basics
Education
About Bayer
©2018 Bayer, Shawnee Mission, Kansas 66201. Bayer and the Bayer Cross are registered trademarks of Bayer.
You’re leaving BayerDVM.
Bayer isn't responsible for the content of other linked websites. Are you sure you want to leave the site?
Antimicrobial Activity of Enrofloxacin Against Staphylococcus Intermedius Strains Isolated From Canine Pyodermas (Pages 171–175)
Related Interests
Rating and Stats
Sharing Options
Document Actions
Pages 2 to 5 are not shown in this preview.
Recommended Documents
Documents Similar To Antimicrobial Activity of Enrofloxacin Against Staphylococcus Intermedius Strains Isolated From Canine Pyodermas (Pages 171–175)
Documents About Antimicrobial Resistance
More From jen
Footer Menu
Legal
- Help / FAQ
- Accessibility
- Purchase help
- AdChoices
- Publishers
Social Media
- Copyright © 2018 Scribd Inc.
- .
- Browse Books
- .
- Site Directory
- .
- Site Language:
Are you sure?
This action might not be possible to undo. Are you sure you want to continue?
Are you sure you want to delete this list?
Everything you selected will also be removed from your lists.
This book will also be removed from all your lists.
We've curated titles we think you'll love.
The rest of this title will be available soon
Antimicrobial Activity of Enrofloxacin Against Staphylococcus Intermedius Strains Isolated From Canine Pyodermas (Pages 171–175) will be available on
Bivirkninger af Baytril Enrofloxacin
Baytril er varemærket for antibiotikummet enrofloxacin og er godkendt af FDA til brug i kun dyr . Enrofloxacin er klassificeret som et fluorquinolonantibiotisk og er almindeligt ordineret til en bred vifte af bakterielle infektioner hos hunde, herunder åbent sår , luftveje , urinveje og hudinfektioner . Enrofloxacin dræber bakterier ved at hæmme produktionen af bakterierne & amp; # 039; s DNA ifølge PetPlace.com . Som med al medicin , er enrofloxacin have nogle bivirkninger . Salg Allergier Salg
Allergi over for antibiotika er en anledning til bekymring, og en almindelig bivirkning af Baytril . Tegn på en allergisk reaktion omfatter , & quot; hævelse i ansigtet , nældefeber , skrabe , pludseligt indsættende diarré , opkastning, chok , krampeanfald, blege gummer, kolde lemmer , eller koma , & quot; Ifølge læger Foster og Smith Apotek . Hunde , der udviser nogen af disse symptomer, bør tages til dyrlægen med det samme.
Hunde med præ- eksisterende lidelser i centralnervesystemet (såsom epilepsi) kan lide øget beslaglæggelse aktivitet når du tager enrofloxacin ; som sådan er enrofloxacin ikke anbefales til disse dyr . Sjældne adfærdsændringer såsom angst, depression , svimmelhed og hallucinationer er også blevet rapporteret .
Baytril kan forårsage appetitløshed , diarré og opkastning. Dette kan være direkte relateret til en allergi over for medicin eller en separat bivirkning af antibiotika selv. Læger Foster og Smith anbefale have masser af drikkevand til rådighed for en hund tager Baytril at sikre, at det ikke bliver dehydreret , en tilstand forværres af opkastning og diarré.
Hunde tager antacida eller andre former for mave beskyttere eller jern bør ikke tage enrofloxacin , advarer PetPlace.com . Enrofloxacin kan interagere negativt med disse typer af medicin og bør kun kombineres under tilsyn af hunden & amp; # 039; . S dyrlæge
Unge hunde i alderen fra bør ikke gives 4 til 28 uger Baytril grundet potentiel ledbrusk skader. Hvalpe tager Baytril kan udvikle hævelse i deres samlinger og bliver halt , ifølge PetPlace.com . Kun i sjældne tilfælde , og under streng veterinær kontrol , bør en hvalp gives Baytril .
Relaterede artikler
Hvad er hot
Strukturen i æselører
Hjørnetænder har fremragende hørelse , delvist på grund af L- formen af deres øregang som hjælper med at beskytte trommehinden . Denne struktur gør det også sværere for voks eller fremmede stoffer til at forlade øre og kan resultere i vanskeligheder med at høre , selv døvhed. Visse racer er genetisk disponerede for høreproblemer , og andres store mængder af hår gør dem også mere tilbøjelige til at udvikle øre maladies . Anatomi strukturen i en hunds øre er kompleks. En hunds øre er opdelt i
Hvorfor har min hvalp Hold Spitting Up Vand
Hvalpe spytte vand op af flere grunde - ? Mest almindeligt er hvalpen drikke for hurtigt og kan ikke fordøje det beløb, han drikker . Hvalpe , der drikker stærkt efter træning kan også spytte op . En hvalp har brug for mere vand end en voksen hund , især hvis han spiser en tør foderpille kost. Du kan styre, hvornår din hvalp drikkevarer , men vær sikker på at han får så meget vand i løbet af en dag, som han ønsker. For at udelukke medicinske spørgsmål , bør en hvalp , der spytter vand op jævnlig
Naturlig Repellent for Dogs
Naturlig insektmiddel er et godt alternativ til kemiske afskrækningsmidler , fordi det er sikkert , ikke-giftige og har ingen bivirkninger . Naturlige olier er effektiv mod myg, lopper og flåter , men er sikkert for børn og voksne så godt. Betydning Insektbekæmpelse vil afskrække lopper og inficerede myg , der indeholder hjerteorm larver og West Nile-virus . Time Frame Anvend naturlige insektmiddel til din hund efter badning , før de går ind i skoven og i skumringen , når myg er mere tilb
hund Sundhed
Special hot
Copyright Kæledyr alle rettigheder forbeholdes
Antimicrobial Activity of Enrofloxacin Against Staphylococcus Intermedius Strains Isolated From Canine Pyodermas (Pages 171–175)
Related Interests
Rating and Stats
Sharing Options
Document Actions
Pages 2 to 5 are not shown in this preview.
Recommended Documents
Documents Similar To Antimicrobial Activity of Enrofloxacin Against Staphylococcus Intermedius Strains Isolated From Canine Pyodermas (Pages 171–175)
Documents About Antimicrobial Resistance
More From jen
Footer Menu
Legal
- Help / FAQ
- Accessibility
- Purchase help
- AdChoices
- Publishers
Social Media
- Copyright © 2018 Scribd Inc.
- .
- Browse Books
- .
- Site Directory
- .
- Site Language:
Are you sure?
This action might not be possible to undo. Are you sure you want to continue?
Are you sure you want to delete this list?
Everything you selected will also be removed from your lists.
This book will also be removed from all your lists.
We've curated titles we think you'll love.
The rest of this title will be available soon
Antimicrobial Activity of Enrofloxacin Against Staphylococcus Intermedius Strains Isolated From Canine Pyodermas (Pages 171–175) will be available on
BAYER
PACK 3 X BAYTRIL FLAVOUR 50 MG FÜR KATZEN UND HUNDE 10 TABLETTEN
Antiinfektivum zur Behandlung von bakteriellen Einzel- oder Mischinfektionen der Atmungs- und Verdauungsorgane, der Harnwege, der Haut sowie von Wunden bei Katzen und Hunden
- BELGIEN : Verschreibungspflichtig
- BULGARIEN : Verschreibungspflichtig
- DEUTSCHLAND : Verschreibungspflichtig
- DÄNEMARK : Verschreibungspflichtig
- ESTLAND : Verschreibungspflichtig
- FINNLAND : Verschreibungspflichtig
- FRANKREICH : Verschreibungspflichtig
- GRIECHENLAND : Verschreibungspflichtig
- IRLAND : Verschreibungspflichtig
- ITALIA : Verschreibungspflichtig
- KROATIEN : Verschreibungspflichtig
- LETTLAND : Verschreibungspflichtig
- LITAUEN : Verschreibungspflichtig
- LUXEMBURG : Verschreibungspflichtig
- MALTA : Verschreibungspflichtig
- MONACO : Verschreibungspflichtig
- POLEN : Verschreibungspflichtig
- PORTUGAL : Verschreibungspflichtig
- RUMÄNIEN : Verschreibungspflichtig
- SCHWEDEN : Verschreibungspflichtig
- SLOWAKEI : Verschreibungspflichtig
- SLOWENIEN : Verschreibungspflichtig
- TSCHECHISCHE REPUBLIK : Verschreibungspflichtig
- UNGARN : Verschreibungspflichtig
- VEREINIGTES KÖNIGREICH GROßBRITANNIEN : Verschreibungspflichtig
- ZYPERN : Verschreibungspflichtig
- ÖSTERREICH : Verschreibungspflichtig
Baytril flavour 50 mg Tablette für Katzen und Hunde


Комментариев нет:
Отправить комментарий