Это видео недоступно.
Очередь просмотра
- Удалить все
- Отключить
Nana the Border Collie Performs Amazing Dog Tricks
Хотите сохраните это видео?
- Пожаловаться
Пожаловаться на видео?
Понравилось?
Не понравилось?
Текст видео
Nana is available for print ads, commercials, film, and other media work. If you are interested in using her for your project, please contact us: http://www.useyourclicker.com/need-a-.
The music contained in this video is the work of Josh Woodward, whose music can be found at the following website:
Nana the Border Collie dog clicker training waving salute trick leg cock leglifting fake peeing walking on two legs standing on back hind legs walking like a human person blowing bubbles limping with back leg limp trick spinning in circles tuck yourself in bang play dead cover nose shame Border Collie walking handstand walking on front feet hand stand in motion canine footstall standing on trainer's feet shoes parking crawling skateboarding roll yourself into a blanket trick head down cross paws cane trick dog dancing disc dog frisbee say your prayers cop cop your feet on mine take off my socks shake head on cue tidy up your toys backwards circles backwards figure 8s backwards leg weaving get the paper untie shoes hold sign kennel up NanaBorderCollie cute puppies dog training puppies the smartest dog in the world the world's smartest dog the smartest dog on earth Nana the wonder dog the most amazing dog tricks compilation
Naturally Treating Your Dog’s Urinary Tract Infection

The discomfort and irritation of a urinary tract infection is very uncomfortable and painful for your dog.
If you’ve ever had one yourself, then you know how uncomfortable these infections are.
As with many conditions that impact humans, pet owners are often unaware that their dog has a urinary tract infection until they see blood in the urine and then the concern grows.
Causes and Concerns
There are a number of things that can cause a urinary tract infection in your dog, including:
- E-coli bacteria
- Bladder inflammation and/or infection
- Trauma
- Stress
- Cancer
- Prostate disease
- Spinal cord difficulties and abnormalities
- Incontinence
- Stones or debris from the bladder or urethra
- Congenital abnormalities
- Hormonal issues
There are also a number of risk factors for a urinary tract infection. It most often occurs in older female dogs as well as pooches with diabetes.
The most commonly occurring disease in dogs over the age of seven years is incontinence, as leakage can occur due to a weaker urinary sphincter muscle. Dogs with conditions like adrenal disease are also more predisposed to urinary tract infections.
Symptoms of Urinary Tract Infections in Dogs
Figuring out if your dog has a urinary tract infection is a matter of observation. As is always the case with issues surrounding your pooch’s health, it’s important to check with holistic veterinarian to not only confirm the condition you suspect but to confirm the adequacy of treatment options for your dog.
Some of the signs and symptoms associated with urinary tract infections in dogs include:
- Bloody or cloudy urine
- Fever
- Loss of bladder control
- Inability to urinate
- Greater frequency of urination
- Crying or straining to pass urin
- Vomiting,
- Lethargy
- Weight loss
- Back pain
- Appetite changes
- Strong odor in uirne
- Increased consumption of water
As the aforementioned symptoms can relate to other conditions, it’s important to get a solid diagnosis from a trained professional if the symptoms persist.
 Treatment
Treatment
There’s been some research done and there are a number of possibilities that you can explore. Because we like to advocate natural treatment options as often as possible, our focus leans to those areas.
Often times dogs are on and off antibiotics with no resolve to the real problem. Surgery, in some extreme cases, may be considered as well.
Our own topical Probiotic Spray, as seen here, works wonders for dogs and cats with UTI’S. You spray it directly to the vagina/uretra on females or the penis area of male dogs. It’s very convenient and effective because it’s applied directly to the problem areas.
Other Helpful Treatment Ideas …
Some have advocated for starting a treatment regimen with cranberry juice, which has been commonly cited as aiding in keeping bacteria away from the bladder. In humans, cranberry is a key in prevention and support of urinary health. In dogs, it can have similar effects but it doesn’t always do the trick alone.
D-Mannose, which is a naturopathic remedy often used to treat urinary tract infections including reoccurring infections caused by a genetic imperfection. D-Mannose has been known to disrupt the ability of e-coli bacteria from sticking to the urinary tract.
It is derived from mannose, which is a sugar molecule (binding). Some have reported combining cranberry with a dosage of D-Mannose and have seen great improvements in their dogs’ urinary tract conditions.
The best forms are those that include cranberry extract. You have to be patient when using D-Mannose. Don’t expect immediate results.
Check with your vet first regarding using D-Mannose if your dog is a diabetic.
Warning: DO NOT buy brands that include Xylitol which is deadly to dogs.
Dosage Guide: a 15-1/2 lb. dog would roughly get a little less than 1/4 teaspoon mixed into food with a little water.
It’s always a good idea to include a probiotic to speed up the healing process.



yes, how many times a day? Thanks!
Not sure what you’re asking Kathy?
If referring to D Mannose, the dosage is in the article.
Hi Could you tell me where i can purchase the d-mannose that you feature in your article or an equivalent product. thks
If you click on the image in the article, it will take you to Amazon where you can purchase it. However, I see that you are in the UK, so the following link and product might work better for you.
I hope it helps Steve.
OK. Great. A 15 1/2 pound dog would get a little less than a teaspoon. Once a day? Twice day? I have the same question Kathy had.
Please advise-if the dog’s Urine PH is 4 which is very acidic, is it still okay to give the D-Mannose to the dog? Dog has e-coli and no antibiotic is killing it.
What do you feed your dog Joy? Do you include any supplements?
A dog’s pH should be between 6 and 6.5 but not above 7. Let me know about the supplements and what else you’re doing? Diet matters.
Why will they not answer the questions about how many times a day is the dosage stated above. Very frustrating due to the fact I just bought this stuff and have no idea how much to give my dog.
What exactly did you buy Patti and from where, because we don’t show your email address in any of our orders?
Overview of Enteric Campylobacteriosis
By Alicja E. Lew-Tabor, BSc (Hons), PhD, Principal Research Fellow, Queensland Alliance for Agriculture & Food Innovation, The University of Queensland
- Enteric Campylobacteriosis
Campylobacter spp are spiral, microaerobic, gram-negative bacteria that cause gastroenteritis in people and animals. Several Campylobacter spp are zoonotic. Many domestic animals develop acute gastroenteritis after ingestion of Campylobacter spp, including dogs, cats, calves, sheep, pigs, ferrets, mink, monkeys, and several species of laboratory animals. (See also Bovine Genital Campylobacteriosis, see Zoonotic Diseases, and see Avian Campylobacter Infection.) Infection with C jejuni is one of the most common causes of gastroenteritis in people worldwide and is the most extensively studied Campylobacter species.
Campylobacter spp are spiral or curved rods that exhibit a characteristic corkscrew darting motility, mediated by a single polar flagellum. These are slow growing, with a generation time of
90 min, fastidious, and require enriched medium and microaerobic conditions with increased CO2 (3%–15% O2, 3%–10% CO2, 85% N2) for growth.
The family Campylobacteraceae consists of three genera, including Campylobacter and Arcobacter associated with animal and human diseases. Certain species are present commensally in animals as suspected reservoirs for human infections. The thermophilic Campylobacter spp, C jejuni, or C coli have the highest prevalence and disease impact. Campylobacter species causing diseases in livestock include C jejuni subsp jejuni (enteritis and abortion), C coli, C mucosalis (porcine enteritis), C upsaliensis, C helveticus (companion pet enteritis), C hyointestinalis subsp hyointestinalis (porcine and bovine enteritis), C sputorum (abortions in sheep), and C fetus subsp fetus (isolated from intestinal tracts of sheep and cattle, sporadic abortions). Certain species such as C jejuni, C hyointestinalis, and C fetus possess closely related subspecies with different disease foci. Initially, Arcobacter spp were considered to be aerotolerant campylobacters and are implicated in reproductive disorders, mastitis, gastric ulcers, and/or diarrhea in livestock, including A cryaerophilus (previously C cryaerophila), A skirrowii, A thereius, and A butzleri.
Transmission and Epidemiology:
Transmission is food- or waterborne or via fecal-oral spread. Animals serve as reservoir hosts for Campylobacter spp infections in both animals and people throughout the world. The predominant ecologic niche for Campylobacter spp is the GI tract of a wide variety of domesticated and wild vertebrates, and zoonotic transmission from animals to people in meat of animal origin, especially chicken, is a food safety issue. Campylobacter spp are also commonly isolated from free-living birds, including migratory birds and waterfowl, crows, gulls, and domestic pigeons, which can contaminate environments of grazing animals. Wild rodents and insects such as flies have also been reported to harbor and transmit C jejuni. Fecal contamination of the environment provides a ubiquitous source of these organisms under appropriate conditions for their survival. Campylobacter spp can persist for long periods in feces, milk, water, and urine, especially at temperatures close to 4ºC. In adverse conditions, C jejuni jejuni converts to a viable nonculturable form that can be reactivated when ingested.
Human foods documented as contaminated with Campylobacter include chicken, turkey, beef, pork, fish, and milk. Domesticated poultry are the most significant reservoir of C jejuni jejuni for people, causing 50%–70% of cases; chicken meat is the number one source. Dogs and cats are commonly infected similar to their owners when they ingest undercooked poultry.
Pathogenesis:
Bacterial motility, mucus colonization, toxin production, attachment, internalization, and translocation are among the processes associated with C jejuni jejuni virulence. Infection begins with ingestion of C jejuni jejuni in contaminated foods or water. Gastric acid provides a barrier, and the bacteria must reach the small and large intestines to multiply; C jejuni invades both epithelial cells and cells within the lamina propria.
Clinical Findings:
Abdominal pain, fever, diarrhea, blood in feces, and inflammatory cells in feces demonstrate the inflammatory nature of the infection. Natural infections with C jejuni jejuni resulting in enteritis have been reported in juvenile macaques, weaning-age ferrets, dogs, cats, and swine. Chickens, rodents, ferrets, primates, rabbits, and pigs have been inoculated experimentally by various routes with C jejuni and subsequently developed enteritis. Clinical reports describe primary infections with systemic spread, infection with mucosal disease, infection without disease but with short-term bacterial persistence, and infection with resistance and no bacterial persistence. These reports support the idea that C jejuni jejuni produces a spectrum of disease scenarios, depending on the immune status of the host, bacterial virulence, gene expression, and other factors.
C jejuni jejuni, C coli, C jejuni, C upsaliensis, and C helveticus are the Campylobacter spp that have been associated with intestinal disease in companion animals. C jejuni jejuni causes diarrhea in dogs and cats, which are considered a significant source of the bacterium for the human population. Diarrhea is usually acute but can be recurrent. Diarrhea lasting 515 days is the most common clinical sign in dogs
C jejuni can stably colonize the small and large intestines, although most animals show cecal and colonic lesions with typhlocolitis. In swine and mice, gross lesions observed in C jejuni enteritis include enlarged and fluid-filled ceca and proximal colons with thickened walls. Lymph nodes (ileocecocolic and mesenteric) draining infected sites become significantly enlarged. Infection with particular strains of C jejuni produces bloody exudates with mucus. Histopathologic features include a marked inflammation of the lamina propria, dominated by neutrophilic polymorphonuclear cells and mononuclear cells that sometimes extend into submucosa. Immune cells such as plasma cells, macrophages, and mononuclear cells have been found in smaller numbers in the lamina propria. Damage to, sloughing of, and ulceration of the epithelial surface and edema have also been seen in most infected species. In pigs and mice, damage to the epithelial surface is associated with the presence of C jejuni at the basolateral surface of the epithelium, in paracellular junctions of the epithelium, and in erosive and ulcerative lesions of the epithelium; there is often a mucopurulent neutrophilic exudate with sloughed and lysed epithelial cells and erosive or ulcerative lesions where C jejuni is associated with the basolateral aspect of sloughing villous tip cells in the colon. Crypt abscesses and damage to the crypt epithelium are also common findings.
Campylobacter spp can be found in both healthy and diarrheic animals; thus, clinical signs and postmortem findings depend on the species and the host animal and its age. Diagnosis of enteric campylobacteriosis relies on isolation of the causative agent using selective media under microaerophilic conditions. Fresh fecal samples should be collected and transported to the laboratory preferably on the same day and within at least 2 days for processing. If transport to the laboratory is delayed, transport media and storage at 4°C produce the best results. Campylobacters are very sensitive to environmental conditions, including dehydration, atmospheric oxygen, sunlight, and increased temperature. Organisms are thin (0.2–0.8 µm × 0.3–5 µm), gram-negative, motile, curved rods. The cells are S-shaped or curved but are occasionally long (8 µm) spiral rods. They exhibit a typical spiraling motility. In unfavorable growth conditions, spiral rods undergo a degenerate conversion to coccoid forms. Campylobacters can be quickly outgrown by contaminating microbes during prolonged transport to the laboratory, and isolation of pure colonies for downstream testing can be difficult. Filtration using 0.45 µm filters can help because campylobacters will pass through.
Enrichment is required for most clinical sampling unless material can be transported to the laboratory immediately. When samples are collected in swabs, the use of commercially available transport tubes containing medium, such as Amies, is recommended. The medium can be plain agar or charcoal-based. Several transport media have been described for transport of fecal specimens, including Cary-Blair, modified Cary-Blair, modified Stuart medium, Campy thioglycolate medium, alkaline peptone water, and semisolid motility test medium. Other media are recommended for the isolation of campylobacters associated with reproductive losses.
Campylobacter spp do not ferment carbohydrates, and other biochemical characteristics are thus used to identify different species. Thermophilic/thermotolerant Campylobacter spp, including C jejuni jejuni, C coli, C upsaliensis, C lari, C mucosalis, C sputorum, C hyointestinalis, and C helveticus grow best at 42°C, although they are capable of growth at 37°C. C fetus do not grow or grow poorly at 42°C. Alternatively, this species grows well at 25°C, whereas the thermophilic campylobacters do not (except C mucosalis, which can grow at 42° and 25°C, weak growth for C hyointestinalis at 25°C). C jejuni is differentiated on its ability to hydrolyze hippurate, and C upsaliensis has negative or weak catalase production and is differentiated from other campylobacters because of its sensitivity to nalidixic acid. C helveticus is also catalase negative but can be difficult to differentiate biochemically from C upsaliensis relying on distinctive colony morphologies.
Differentiation of subspecies can be necessary for identification of significant pathogens. C jejuni subsp jejuni is the main cause of enteritis, whereas C jejuni subsp doylei has been isolated only from enteritis cases of children and not animals. They can be differentiated by the ability of C jejuni doylei to reduce nitrate. Similarly, C hyointestinalis subsp hyointestinalis can cause bovine and porcine enteritis; however, C hyointestinalis subsp lawsonii has been isolated from the porcine stomach, but it is not known to cause disease. The subspecies can be differentiated by testing the intolerance of C hyointestinalis lawsonii to 1.5% bile and/or 0.1% potassium permanganate.
Arcobacter spp (previously known as aerotolerant campylobacters) can also be associated with human and animal diarrhea and with animal abortions. Arcobacters are usually not thermophilic but can be confused with the nonthermophilic Campylobacter spp if aerotolerance is confirmed using standardized suspensions of organisms. Although most cases of human enteritis are attributed to C jejuni jejuni, C coli, C lari, and C upsaliensis, it has been suggested that the importance of other species also associated with GI illness may be significantly underdiagnosed as a consequence of inappropriate isolation and identification methods.
Immunodiagnosis (ELISA) is unsuitable to diagnose intestinal Campylobacter infections.
PCR-based methods effectively identify infection, especially if cultivation is difficult or if the sample has been somewhat mishandled. However, a positive test is not sufficient evidence to determine causation and must be considered in conjunction with clinical signs.
Treatment and Control:
Clindamycin , gentamicin , tetracyclines, erythromycin , cephalosporins (eg, cephalothin), and fluoroquinolones (eg, nalidixic acid) are effective against C jejuni, C helveticus, and C upsaliensis. C fetus, C hyointestinalis, C mucosalis, and C sputorum are usually resistant to the fluoroquinolones yet sensitive to cephalosporins. C coli are sensitive to fluoroquinolones but resistant to cephalosporins. Susceptibilities to penicillins and trimethoprim are variable across Campylobacter spp. Resistance to the fluoroquinolones, tetracycline , kanamycin , and some other antibiotics has been documented among the Campylobacter spp, mediated by both chromosomal and plasmid mechanisms. Culture-dependent diagnosis can provide isolates for antibiotic sensitivity testing. However, some animals remain colonized and become persistent shedders despite antibiotic therapy. If the goal of treatment is to decrease the risk of zoonotic transmission to a susceptible household member, antibiotic treatment alone may be inadequate. Control involves treatment, removal to a clean environment, and prospective fecal testing to ascertain shedding status; even so, low infective doses and the ubiquitous distribution of the organism pose significant challenges.
Resources In This Article
- Enteric Campylobacteriosis
Was This Page Helpful?
Also of Interest
Test your knowledge
Ruminants (cattle, sheep, and goats) lack which of the following teeth?
Merck and the Merck Veterinary Manual
Merck & Co., Inc., Kenilworth, NJ, USA is a global healthcare leader working to help the world be well. From developing new therapies that treat and prevent disease to helping people in need, we are committed to improving health and well-being around the world. The Merck Veterinary Manual was first published in 1955 as a service to the community. The legacy of this great resource continues as the Merck Veterinary Manual in the US and Canada and the MSD Manual outside of North America.
© 2018 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA
Coli hund
- gram negative bacteria -
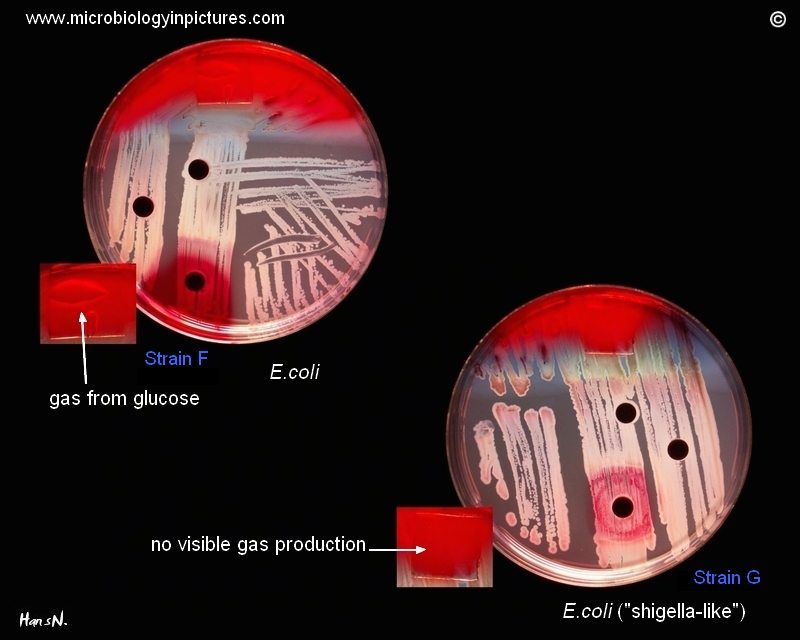
Some "lactose negative" strains of E.coli (the late lactose fermenters) can mimic Shigella spp., especially in cases that they produce only little amount of gas from glucose (Strain G).
General description of testing on Endo agar with biochemical slope is here.
Coli hund
Purchase PDF Purchase

Veterinary Microbiology
Highlights
Some E. coli pathogenic strains may cause enteric or extra-intestinal disease.
We examine high puppy mortality due to extra-intestinal pathogenic E. coli.
Bitch's milk could be the main source of ExPEC infection.
Isolated E. coli strains were assigned to phylogroup B2.
Strains were biofilm producers with ability to colonize and survive in the host.
A 10-day-old litter of five puppies of Bracco Italiano dog breed showed weakness and diarrhea and, 2 days later, four of them died. At the same time, the bitch showed high hyperthermia (40 °C) and endometritis. The necropsy of a puppy revealed a severe lobar pneumonia accompanied with a bilateral nephrosis. No gross lesions were detected in other organs. Histopathology of the lung revealed severe multifocal fibrino-suppurative necrotizing bronchiolar-alveolitis associated with rod-shaped bacterial aggregates and diffuse interstitial lymphocytic infiltration. The kidney showed severe multifocal necrosis of the tubular epithelium and diffuse severe congestion of the parenchyma. A pure culture of hemolytic Escherichia coli carrying the Cnf-1 gene was identified, from both the puppy organs and bitch's milk. Moreover, phylo-typing assigned them to the phylogroup B2. Two weeks later, fecal samples from the bitch and the survived puppy were collected for a second microbiological analysis, identifying two hemolytic E. coli strains, Cnf positive and Cdt negative and Cnf and Cdt negative, respectively.
Some E. coli pathogenic strains may cause enteric or extraintestinal disease. In dogs and cats, strains of extraintestinal pathogenic E. coli (ExPEC) produce specific virulent factors such as hemolysis and cytotoxin necrotizing factors (Cnf). In this episode, we hypothesize that the bitch's milk could be the main source of ExPEC infection causing high puppies mortality. The role of the bitch as a carrier could not be excluded: stressful conditions, such as pregnancy and delivery, would change the host–pathogen dynamics possibly increasing the release of the infectious burden.
Choose an option to locate/access this article:
Check if you have access through your login credentials or your institution.
Coli hund
Purchase PDF Purchase

Molecular and Cellular Probes
Escherichia coli isolates from all surveillance patients ⩽ 20 months of age seen for diarrhoea at the Dhaka Clinical Treatment Facility of the International Centre for Diarrhoeal Disease Research, Bangladesh between March 1 and August 31, 1988, were collected and hybridized with DNA probes to assess the potential importance of diarrhoeagenic E. coli among paediatric patients in Bangladesh. of 396 patients evaluated, 18% were infected with enteropathogenic E. coli (EPEC) adherence factor (EAF)-positive E. coli, 23% were infected with enterotoxigenic E. coli (ETEC), 9% were infected with Shiga-like toxin-positive E. coli, and 13% were infected with diffuse adhesiveness-positive E. coli. None were infected with enteroinvasive E. coli. Ten percent of patients were colonized with more than one type of potential diarrhoeagenic E. coli. The majority of EAF-positive isolates were of traditional EPEC O:H serotypes. Although this was not a case-control study, the large number of EPEC and ETEC, which are recognized enteric pathogens, suggests these organisms are important causes of diarrhoeal diseases in this pediatric population.
Choose an option to locate/access this article:
Check if you have access through your login credentials or your institution.
Gut Pathogens
Table of Contents
A novel enzyme-link immunosorbent assay for detection of Escherichia coli O157:H7 using immunomagnetic and beacon gold nanoparticles
- Zhiqiang Shen 1 ,
- Nannan Hou 1 ,
- Min Jin 1 ,
- Zhigang Qiu 1 ,
- Jingfeng Wang 1 ,
- Bin Zhang 1 ,
- Xinwei Wang 1 ,
- Jie Wang 2 ,
- Dongsheng Zhou 2Email author and
- Junwen Li 1Email author
© Shen et al.; licensee BioMed Central Ltd. 2014
This paper presents a functional nanoparticle-enhanced enzyme-link immunosorbent assay (FNP-ELISA) for detection of enterohemorrhagic Escherichia coli (EHEC) O157:H7. Immunomagnetic nanoparticles (IMMPs) conjugated with monoclonal anti-O157:H7 antibody were used to capture E. coli O157:H7. Beacon gold nanoparticles (B-GNPs) coated with polyclonal anti-O157:H7 and biotin single-stranded DNA (B-DNA) were then subjective to immunoreaction with E. coli O157:H7, which was followed by streptavidin-horseradish peroxidase (Strep-HRP) conjugated with B-GNPs based on a biotin-avidin system. The solutions containing E. coli O157:H7, IMMPs, B-GNPs, and Strep-HRP were collected for detecting color change. The signal was significantly amplified with detection limits of 68 CFU mL -1 in PBS and 6.8 × 10 2 to 6.8 × 10 3 CFU mL -1 in the food samples. The FNP-ELISA method developed in this study was two orders of magnitude more sensitive than immunomagnetic separation ELISA (IMS-ELISA) and four orders of magnitude more sensitive than C-ELISA. The entire detection process of E. coli O157:H7 lasted only 3 h, and thus FNP-ELISA is considered as a time-saving method.
Introduction
The World Health Organization estimated that about 1.8 million people worldwide die every year from diarrheal diseases, which are often caused by consuming microbiologically contaminated food or by drinking water [ 1 ]. Among the pathogens causing diarrheal diseases, enterohemorrhagic Escherichia coli (EHEC) strains are prominently responsible for serious foodborne outbreaks [ 2 , 3 ]. In particular, E. coli O157:H7, a predominant strain of EHEC that was first isolated and recognized as a new type of intestinal pathogenic bacterium in the United States in 1982 [ 4 ], has become a global public health problem. E. coli O157:H7 outbreaks have occurred in many developing and developed countries, causing huge health care costs and product recalls. The Center for Disease Control and Prevention of the United States estimated that 73,000 cases of illness and 61 deaths per year in the United States are caused by E. coli O157:H7 [ 5 ].
The development of a rapid and reliable detection of E. coli O157:H7 has become highly important for food safety and public health [ 6 ]. However, traditional methods for the detection of E. coli O157:H7 encompassing enrichment, plating, culturing, enumeration, biochemical testing, and microscopic examination can take up to 60 h, thereby being laborious and time-consuming [ 7 ]. Polymerase chain reactions (PCRs), including simple PCR [ 8 ], multiplex PCR [ 9 , 10 ], and real-time PCR [ 11 , 12 ], are commonly used for rapid detection of E. coli O157:H7, but require complex set-ups and well-trained personnel. In addition, some very sensitive and selective but expensive, complicated, and time-consuming methods have been applied in the detection of E. coli O157:H7, especially including immunomagnetic separation (IMS) analysis [ 13 ], flow cytometry [ 14 ], fluorescence in situ hybridization [ 15 ], DNA microarrays [ 16 ], and several label-free methods (such as surface plasmon resonance [ 17 ] and use of electrochemical impedance immunosensors [ 18 , 19 ]).
Enzyme-link immunosorbent assay (ELISA) was reported to quantitatively detect immunoglobulin G in 1971 [ 20 ]. Conventional ELISA (C-ELISA) has high reproducibility and possibility for the simultaneous quantification of a great number of assays, and is widely used to detect the presence of substances, including bacteria [ 21 ], viruses [ 22 ], proteins [ 23 ], and pesticides [ 24 ]. However, the detection limit of C-ELISA to E. coli O157:H7 is only 10 5 to 10 7 CFU mL -1 [ 25 ], which is inadequate when the infectious dose is lower than 100 cells [ 26 ].
In recent years, the emergence of nanotechnology is opening new horizons for high detection limits in biological fields [ 27 – 30 ]. Nanoparticles of various shapes, sizes, and compositions have broad applications in microorganism detection [ 31 , 32 ]. Much attention has been focused on amplifying the detection signal using nanoparticles [ 33 , 34 ], which can enhance enzyme activity [ 35 , 36 ]. Magnetic and gold particles have been used to improve the detection limit of ELISA [ 30 , 37 ].
In this study, we developed a functional nanoparticle-enhanced ELISA (FNP-ELISA) using immunomagnetic nanoparticles (IMMPs) and beacon gold nanoparticles (B-GNPs) for detecting E. coli O157:H7. The detection limit of E. coli O157:H7 by the developed FNP-ELISA is much higher than that of C-ELISA or immunomagnetic separation ELISA (IMS-ELISA), and thus FNP-ELISA had the highest sensitivity compared to the other ELISA methods.
Materials and methods
Reagents and materials
Rabbit polyclonal anti-E. coli O157:H7 antibody and mouse monoclonal anti-O157:H7 antibody were prepared and purified in our laboratory. Single-stranded DNA 5′(biotin)-GCTAGTGAACACAGTT-GTGTAAAAAAAAAA (SH)-3′ was synthesized by Sangon Biotech Co., Ltd. (China). Streptavidin-horseradish peroxidase (Strep-HRP) and peroxidase-conjugated affinipure goat anti-rabbit IgG (IgG-HRP) were purchased from Beijing Biosynthesis Biological Technology Co., Ltd. (China). Bovine serum albumin (BSA), 3,3′,5,5′- tetramethylbenzidine (TMB-H2O2), and hydrogen tetrachloroaurate (III) trihydrate (HAuCl4 · 3H2O, 99.9%) were purchased from Sigma-Aldrich (USA). Dextran with a molecular weight of 40,000 (T-40) was obtained from Pharmacia (GE Healthcare, USA). Sorbitol-MacConkey agar (SMAC) and xylose-lysine-tergitol 4 (XLT4) agar were purchased from Difco (Becton Dickinson, USA). Ferric chloride hexahydrate (FeCl3 · 6H2O), ferrous chloride tetrahydrate (FeCl2 · 4H2O), and other chemicals were of analytically pure grade or better quality. The buffer solutions were prepared in our laboratory. All aqueous solutions were prepared using ultrapure water (18.0 MΩ/cm) as required.
Preparation of microbial samples
E. coli O157:H7 strain 35150 and E. coli K12 were obtained from the American Type Culture Collection (ATCC, USA). Salmonella senftenberg 50315, Shigella sonnei 51081, and E. coli O157:Hund strain 21531 (Hund indicated that H antigen was not determined) [ 38 ] were obtained from the Institute of Epidemiology and Microbiology, Academy of Preventive Medical Sciences of China. Pure cultures of bacteria were grown in nutrient broth at 37°C for 24 h before use. The concentrations of E. coli O157:H7, O157:Hund, and K12 were determined by the conventional surface plate count method using SMAC. S. senftenberg and S. sonnei were enumerated using XLT4 agar. The cultured bacteria were divided into two portions. The first portion was placed in a boiling water bath for 20 min to kill the bacterial cells, and diluted to the desired concentration with PBS (0.01 M, pH 7.4) for ELISA detection. The second portion was not heated because the number of living cells was counted.
Milk, vegetable, and ground beef were purchased from a local market in Tianjin (China), and each weighed 25 g (mL) for detection. The killed E. coli O157:H7 solution was transferred into a small vial equipped with an atomizer. The mists of E. coli O157:H7 inoculums were sprayed onto the three samples, and the samples were then stored at 4 ± 1°C for 1 h. Each sample was added to 0.25 mL of E. coli O157:H7 solution. The samples were placed into sterile filter stomacher bags, and macerated in 225 mL of PBS with a stomacher blender (Bilon-8 Bilang Co. Ltd., Beijing, China) at 200 rpm for 2 min. The homogenate was serially diluted in PBS for ELISA detection. The negative samples that were not added to E. coli O157:H7 solution were analyzed according to the Chinese National Standard Method GB/T 4789.36-2008 [ 39 ].
Preparation of IMMPs
Magnetic nanoparticles (MPs) were prepared from FeCl3, FeCl2, ammonia solution, and dextran (T-40), and oxidized with NaIO4 as described previously [ 40 ]. Mouse monoclonal anti-E. coli O157:H7 antibody (0.5 mg/mL) was added to the oxidized MP suspension at a ratio of 0.3:1, mixed thoroughly, and incubated in the dark at 4°C for approximately 24 h. IMMPs were washed three times with PBS by placing a magnetic plate against the side wall of the tubes for 5 min to concentrate the particles into the pellets on the side walls. The supernatant was discarded using a transferpettor. The pellets were resuspended in 1 mL of PBST (0.05% Tween-20 in 0.01 M PBS, pH 7.4). BSA was then added to a final concentration of 1% to block any unreacted or nonspecific site. The amount of IMMPs, incubation time, and separation time varied to determine their effects on the recovery of O157:H7 (details in Supplementary Materials).
Preparation of GNPs
GNPs were prepared according to the literature with slight modifications [ 41 ]. In brief, 2 mL of 1% HAuCl4 was mixed with 198 mL of fresh ultrapure water. The mixture was stirred vigorously with a magnetic agitator while being heated in a boiling water bath for 20 min, followed by the rapid addition of 5 mL of 1% sodium citrate solution. After the color finally turned to full red, the mixture was stirred again for 10 min before cooling to room temperature. The GNP solution was filtered through a 0.22 μm cellulose nitrate filter to remove any floating aggregates. The prepared GNPs were characterized using a transmission electron microscope (TEM; Tecnai G2 F20, FEI, Netherlands) and ultraviolet spectrophotometer (UV 2500, Shimadzu, Japan).
Preparation of various B-GNPs
B-GNPs were prepared following a previously reported procedure [ 42 , 43 ] with slight modifications. Rabbit polyclonal anti-E. coli O157:H7 antibody (7 μg) was added to 1 mL of pH-adjusted GNP solution (pH 8.2) and incubated at room temperature for 30 min. The mixture was added with 30 μL of different concentrations of B-DNA, and incubated in the dark at 4°C for more than 16 h. Approximately 100 μL of 1% sodium chloride solution was then added to the mixture, and incubated at 4°C for 3 h. BSA was added to a final concentration of 1% to block any unreacted or nonspecific site. The prepared B-GNP solution was centrifuged at 20,000 g for 1 h at 4°C. The final deposition was suspended in 0.5 mL of storage buffer (PBS, 0.01 M, pH 7.4, 1% BSA, 0.02% NaN3) and stored at 4°C.
Mouse monoclonal anti-E. coli O157:H7 antibody (100 μL of 5 mg L -1 ) was added to a 96-well plate and incubated at 37°C for 2 h. The plate was rinsed with PBST (0.05% Tween-20 in 0.01 M PBS, pH 7.4) three times to remove unbound antibodies, followed by the addition of 100 μL of PBS-BSA (1% BSA in 0.01 M PBS, pH 7.4) and incubation at 4°C for 12 h. Different concentrations of E. coli O157:H7 (100 μL) were added to each well and reacted at 37°C for 1 h. After rinsing three times, 100 μL of 5 mg L -1 rabbit polyclonal anti-E. coli O157:H7 antibody was added to the plate incubated at 37°C for 1 h. Subsequently, 100 μL of 0.02 mg L -1 IgG-HRP was added to the plate and incubated for 1 h at 37°C. The plate was rinsed three times to remove unbound IgG-HRP. Finally, 100 μL of TMB-H2O2 solution was added to each well and incubated at 37°C for 15 min. The reaction was terminated using 100 μL of 0.5 M sulfuric acid, and the absorbance at 450 nm was measured using a microplate reader.
E. coli O157:H7 was separated using IMMPs according to the previous procedure in Supplementary Materials, and the particle-bacteria complex (100 μL) was finally resuspended. Rabbit polyclonal anti-O157:H7 antibody (100 μL of 5 mg L -1 ) was added to the complex, and incubated at room temperature for 30 min. The unbound antibody was removed by the magnetic plate method. The particle-bacteria-antibody complex was resuspended using 100 μL of 0.02 mg L -1 IgG-HRP, and incubated for 1 h at 37°C. The complex was resuspended and transferred to a 96-well plate after excess IgG-HRP was removed by the magnetic plate method. Finally, TMB-H2O2 and sulfuric acid were subsequently added, and the plate was read at 450 nm using a microplate reader.
IMMPs (10 μL) were added to 1 mL of E. coli O157:H7 suspension at 10 6 CFU mL -1 in a 1.5 mL Eppendorf tube. The tube was carefully inverted several times and incubated at room temperature for 10 min. Approximately 100 μL of the complex of E. coli O157:H7 and IMMPs was obtained by the magnetic plate method. Various B-GNPs (100 μL) were added to 100 μL of the complex, and incubated at room temperature for 30 min. The unbound B-GNPs were removed by the magnetic plate method, and the complex was rinsed three times with PBST. Subsequently, 100 μL of Strep-HRP (0.01 mg L -1 ) solution was added to the Eppendorf tube and incubated at 37°C for 1 h. The unbound Strep-HRP was removed by the magnetic plate method. The final complex was washed three times with PBST by the magnetic plate method, and resuspended in 100 μL of PBS. The suspension was then transferred to a 96-well plate. Finally, TMB-H2O2 and sulfuric acid were subsequently added, and the plate was read at 450 nm using a microplate reader.
Experimental replicates and statistical methods
All the experiments were done with at least biological replicates, and the values were expressed as mean ± standard deviation. A conventionally used positive control to negative control (P/N) value ≥2.1 was considered positive in the three ELISA methods [ 44 ]. When needed, paired Student’s t- test was performed to determine statistically significant differences; P <0.01 was considered to indicate statistical significance.
Results and discussion
Properties of IMMS
We prepared MPs that were roughly spherical in shape with diameters ranging from 40–60 nm, and contained an electron-dense core of 5 nm (Additional file 1 : Figure S1). We also prepared IMMPs, and the recovery of IMMPs increased with increasing amount of IMMPs and incubation time. Relatively high recovery was obtained with 10 μL of IMMPS (Additional file 1 : Table S1) and 10 min of incubation (Additional file 1 : Table S2). The optimal time for separation was 2 min (Additional file 1 : Table S3). This finding was supported by a previous study, which showed that target cells can be separated from samples using MPs coupled with aptamer/nucleic acid/antibody [ 45 , 46 ].
Properties of B-GNPs
Typical TEM images of GNPs.
Experimental design for FNP-ELISA
Flow chart of FNP-ELISA. Preparation of IMMPs and B-GNPs (part A); (part B) comprises five steps, namely, magnetic separation of target cells (step 1), conjugation of B-GNPs (step 2), removal of free B-GNPs (step 3), conjugation of Strep-HRP (step 4), and removal of free Strep-HRP (step 5); and FNP-ELISA detection (part C).
First, functional monoclonal anti-E. coli O157:H7-conjugated IMMPs and polyclonal anti-E. coli O157:H7 antibody and B-GNPs are prepared (A). Second, IMMPs are mixed with a sample to target E. coli O157:H7, and the O157:H7-IMMP complex is separated using the magnetic plate method (step 1, B). Third, B-GNPs are added to target E. coli O157:H7 in the E. coli O157:H7-IMMP complex (step 2, B), and the unbound B-GNPs are removed by the magnetic plate method (step 3, B). Fourth, Strep-HRP is added to react with polyclonal anti-E. coli O157:H7 (step 4, B), and unbound Strep-HRP is removed by the magnetic plate method. Finally, the remaining routine ELISA steps are completed (C).
Optimized amounts of B-DNA, Strep-HRP and B-GNPs for FNP-ELISA
Optimal amounts of B-DNA, Strep-HRP, and B-GNPs. (a) Optimization graph of the B-DNA concentration in the preparation of B-GNPs. (b) Optimization of the B-GNP volume. (c) Strep-HRP volume optimization graph.
The optimal volume of B-GNPs was determined by adding different volumes of the optimized B-GNPs to the complex of E. coli O157:H7 and IMMPs after separating E. coli O157:H7. The detection procedure was then operated as above.
The optimized volume of Strep-HRP was determined when the optimized volume of B-GNPs was applied in the procedure. After optimizing the parameters, various concentrations of E. coli O157:H7 were detected in PBS using FNP-ELISA. The negative samples included K12, S. senftenberg, and S. sonnei in FNP-ELISA. This method was then used to determine the concentration of E. coli O157:H7 in the milk, vegetable, and ground beef samples.
We found that the FNP-ELISA signal increased with increasing B-DNA concentration (Figure 3 a), and the optimal concentration of B-DNA was 40 μmol L -1 . This finding was also observed in a previous study, which reported that excess B-DNA molecules cause excessive HRP molecules to bind with GNPs, posing steric hindrance to hamper antigens from access to antibodies on GNPs [ 47 ].
The signal of FNP-ELISA was strongly dependent on the amount of B-GNPs. The ELISA signal increased as the amount of B-GNPs increased, and reached a plateau at 100 μL (Figure 3 b). Thus, we used 100 μL of B-GNPs in subsequent experiments. The optimal amount of Strep-HRP was also obtained at 100 μL (Figure 3 c).
Comparison of FNP-ELISA to C-ELISA and IMS-ELISA
Functional nanoparticles were used to improve the sensitivity of ELISA, and the results were compared simultaneously. The blank, positive, and negative controls were PBS, E. coli O157:H7 (10 6 CFU mL -1 ), and E. coli O157:Hund (10 6 CFU mL -1 ), respectively. These controls were included on each plate in the experiments.
ELISA Detection sensitivity. The detection limits of C-ELISA, IMS-ELISA, and FNP-ELISA were 6.8 × 10 5 (1.8 ≤ RSD ≤ 4.7), 6.8 × 10 3 (0.6 ≤ RSD ≤ 5.2), and 6.8 × 10 1 CFU mL -1 (0.3 ≤ RSD ≤ 4.7), respectively (n = 3).
The specificity of the three ELISA methods was dependent on the quality of the antibody. The specificity of the rabbit polyclonal anti-E. coli O157:H7 antibody and mouse monoclonal anti-E. coli O157:H7 antibody was tested using 61 bacteria, and false negative and false positive results were not observed (data not shown). E. coli O157:H7 is a common intestinal pathogen, so S. senftenberg and S. sonnei, which are also common intestinal pathogens, were selected as negative samples. E. coli K12, which represented E. coli, was another negative sample. The P/N values of the negative signals were all significantly lower than 2.1 (Figure 4 ), which shows that FNP-ELISA had high specificity.
E. coli O157:H7 was not detected in the raw vegetable, milk, and ground beef samples using GB/T method 4789.36-2008. The detection limits of FNP-ELISA were 6.8 × 10 2 CFU mL -1 in vegetable and milk, and 6.8 × 10 3 CFU mL -1 in ground beef. The reduction in sensitivities may be attributed to the loss of some functional nanoparticles and targeting bacteria in the food residues, particularly grease foods.
Concluding remarks
Multiple ultrasensitive methods for detecting E. coli O157:H7 have been reported [ 48 , 49 ], but they usually require expensive equipment or skilled personnel and thus have difficulty in wide use. C-ELISA has been widely established to detect microorganisms, proteins, pesticides, and heavy metals because of its simplicity and low cost, but its application is limited because of its low detection limit. Data presented here indicated that FNP-ELISA had a high sensitivity in detecting E. coli O157:H7, with a detection limit of 68 CFU mL -1 in PBS and 6.8 × 10 2 to 10 3 CFU mL -1 in foods. The detection limit of FNP-ELISA was about two or four orders of magnitude lower than that of IMS-ELISA or C-ELISA, respectively. Moreover, the total analysis time of FNP-ELISA was only approximately 3 h. Therefore, FNP-ELISA may be used for detection of E. coli O157:H7 in foods, and also for other microorganisms if appropriate antibodies are available.
Declarations
Acknowledgments
This study was supported by National Natural Science Foundation of China (81270041 and 21377061) and Natural Science and Technology Supporting Program of Tianjin (11ZCKFSF01100). The English writing was polished by EnPapers.
Electronic supplementary material
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
DZ and JL conceived and designed the experiments. ZS, NH, MJ, ZQ, JW, BZ, XW and JW performed the experiments. ZS, DZ and JL analyzed the data. ZS, DZ and JL drafted the manuscript. All authors read and approved the final manuscript.
Authors’ Affiliations
References
- Velusamy V, Arshak K, Korostynska O, Oliwa K, Adley C: An overview of foodborne pathogen detection: in the perspective of biosensors. Biotechnol Adv. 2010, 28: 232-254. 10.1016/j.biotechadv.2009.12.004. View ArticlePubMedGoogle Scholar
- Abadias M, Usall J, Anguera M, Solsona C, Vinas I: Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int J Food Microbiol. 2008, 123: 121-129. 10.1016/j.ijfoodmicro.2007.12.013. View ArticlePubMedGoogle Scholar
- Wu CJ, Hsueh PR, Ko WC: A new health threat in Europe: Shiga toxin-producing Escherichia coli O104:H4 infections. J Microbiol Immunol Infect. 2011, 44: 390-393. 10.1016/j.jmii.2011.07.001. View ArticlePubMedGoogle Scholar
- Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, Hebert RJ, Olcott ES, Johnson LM, Hargrett NT, Blake PA, Cohen ML: Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983, 308: 681-685. 10.1056/NEJM198303243081203. View ArticlePubMedGoogle Scholar
- Park S, Kim H, Paek SH, Hong JW, Kim YK: Enzyme-link immuno-strip biosensor to detect Escherichia coli O157:H7. Ultramicroscopy. 2008, 108: 1348-1351. 10.1016/j.ultramic.2008.04.063. View ArticlePubMedGoogle Scholar
- Dweik M, Stringer RC, Dastider SG, Wu Y, Almasri M, Barizuddin S: Specific and targeted detection of viable Escherichia coli O157:H7 using a sensitive and reusable impedance biosensor with dose and time response studies. Talanta. 2012, 94: 84-89. View ArticlePubMedGoogle Scholar
- Fedio WM, Jinneman KC, Yoshitomi KJ, Zapata R, Wendakoon CN, Browning P, Weagant SD: Detection of E. coli O157:H7 in raw ground beef by Pathatrix immunomagnetic-separation, real-time PCR and cultural methods. Int J Food Microbiol. 2011, 148: 87-92. 10.1016/j.ijfoodmicro.2011.05.005. View ArticlePubMedGoogle Scholar
- Johnston LM, Elhanafi D, Drake M, Jaykus LA: A simple method for the direct detection of Salmonella and Escherichia coli O157:H7 from raw alfalfa sprouts and spent irrigation water using PCR. J Food Prot. 2005, 68: 2256-2263. PubMedGoogle Scholar
- Paton AW, Paton JC: Direct detection and characterization of Shiga toxigenic Escherichia coli by multiplex PCR for stx1, stx2, eae, ehxA, and saa. J Clin Microbiol. 2002, 40: 271-274. 10.1128/JCM.40.1.271-274.2002. PubMed CentralView ArticlePubMedGoogle Scholar
- Kumar A, Grover S, Kumar Batish V: Application of multiplex PCR assay based on uidR and fliCH7 genes for detection of Escherichia coli O157:H7 in milk. J Gen Appl Microbiol. 2013, 59: 11-19. 10.2323/jgam.59.011. View ArticlePubMedGoogle Scholar
- Wong LY, Cao Y, Balachandran P, Zoder P, Furtado MR, Petrauskene OV, Tebbs RS: Validation of the Applied Biosystems MicroSEQ real-time PCR system for detection of E. coli O157:H7 in food. J AOAC Int. 2012, 95: 1495-1504. 10.5740/jaoacint.11-289. View ArticlePubMedGoogle Scholar
- Wasilenko JL, Fratamico PM, Narang N, Tillman GE, Ladely S, Simmons M, Cray WC: Influence of primer sequences and DNA extraction method on detection of non-O157 Shiga toxin-producing Escherichia coli in ground beef by real-time PCR targeting the eae, stx, and serogroup-specific genes. J Food Prot. 2012, 75: 1939-1950. 10.4315/0362-028X.JFP-12-087. View ArticlePubMedGoogle Scholar
- Wang L, Li Y, Mustaphai A: Rapid and simultaneous quantitation of Escherichia coli 0157:H7, Salmonella, and Shigella in ground beef by multiplex real-time PCR and immunomagnetic separation. J Food Prot. 2007, 70: 1366-1372. PubMedGoogle Scholar
- Wilkes JG, Tucker RK, Montgomery JA, Cooper WM, Sutherland JB, Buzatu DA: Reduction of food matrix interference by a combination of sample preparation and multi-dimensional gating techniques to facilitate rapid, high sensitivity analysis for Escherichia coli serotype O157 by flow cytometry. Food Microbiol. 2012, 30: 281-288. 10.1016/j.fm.2011.11.002. View ArticlePubMedGoogle Scholar
- Garcia-Armisen T, Servais P: Enumeration of viable E. coli in rivers and wastewaters by fluorescent in situ hybridization. J Microbiol Methods. 2004, 58: 269-279. 10.1016/j.mimet.2004.04.014. View ArticlePubMedGoogle Scholar
- Donhauser SC, Niessner R, Seidel M: Sensitive quantification of Escherichia coli O157:H7, Salmonella enterica, and Campylobacter jejuni by combining stopped polymerase chain reaction with chemiluminescence flow-through DNA microarray analysis. Anal Chem. 2011, 83: 3153-3160. 10.1021/ac2002214. View ArticlePubMedGoogle Scholar
- Wang Y, Ye Z, Si C, Ying Y: Monitoring of Escherichia coli O157:H7 in food samples using lectin based surface plasmon resonance biosensor. Food Chem. 2013, 136: 1303-1308. 10.1016/j.foodchem.2012.09.069. View ArticlePubMedGoogle Scholar
- Wang L, Liu Q, Hu Z, Zhang Y, Wu C, Yang M, Wang P: A novel electrochemical biosensor based on dynamic polymerase-extending hybridization for E. coli O157:H7 DNA detection. Talanta. 2009, 78: 647-652. 10.1016/j.talanta.2008.12.001. View ArticlePubMedGoogle Scholar
- Viswanathan S, Rani C, Ho JA: Electrochemical immunosensor for multiplexed detection of food-borne pathogens using nanocrystal bioconjugates and MWCNT screen-printed electrode. Talanta. 2012, 94: 315-319. View ArticlePubMedGoogle Scholar
- Engvall E, Perlmann P: Enzyme-link immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971, 8: 871-874. 10.1016/0019-2791(71)90454-X. View ArticlePubMedGoogle Scholar
- Chunglok W, Wuragil DK, Oaew S, Somasundrum M, Surareungchai W: Immunoassay based on carbon nanotubes-enhanced ELISA for Salmonella enterica serovar Typhimurium. Biosens Bioelectron. 2011, 26: 3584-3589. 10.1016/j.bios.2011.02.005. View ArticlePubMedGoogle Scholar
- Zhu J, Yang Q, Cao L, Dou X, Zhao J, Zhu W, Ding F, Bu RE, Suo S, Ren Y, Li G, Ren X: Development of porcine rotavirus vp6 protein based ELISA for differentiation of this virus and other viruses. Virol J. 2013, 10: 91-10.1186/1743-422X-10-91. PubMed CentralView ArticlePubMedGoogle Scholar
- Jalallou N, Bandehpour M, Khazan H, Haghighi A, Kazemi B: Evaluation of recombinant sag1 protein for detection of toxoplasma gondii specific immunoglobulin M by ELISA test. Iran J Parasitol. 2012, 7: 17-21. PubMed CentralPubMedGoogle Scholar
- Byer JD, Struger J, Sverko E, Klawunn P, Todd A: Spatial and seasonal variations in atrazine and metolachlor surface water concentrations in Ontario (Canada) using ELISA. Chemosphere. 2011, 82: 1155-1160. 10.1016/j.chemosphere.2010.12.054. View ArticlePubMedGoogle Scholar
- Strachan NJ, Ogden ID: A sensitive microsphere coagulation ELISA for Escherichia coli O157:H7 using Russell’s viper venom. FEMS Microbiol Lett. 2000, 186: 79-84. 10.1111/j.1574-6968.2000.tb09085.x. View ArticlePubMedGoogle Scholar
- Tuttle J, Gomez T, Doyle MP, Wells JG, Zhao T, Tauxe RV, Griffin PM: Lessons from a large outbreak of Escherichia coli O157:H7 infections: insights into the infectious dose and method of widespread contamination of hamburger patties. Epidemiol Infect. 1999, 122: 185-192. 10.1017/S0950268898001976. PubMed CentralView ArticlePubMedGoogle Scholar
- Shen ZQ, Wang JF, Qiu ZG, Jin M, Wang XW, Chen ZL, Li JW, Cao FH: QCM immunosensor detection of Escherichia coli O157:H7 based on beacon immunomagnetic nanoparticles and catalytic growth of colloidal gold. Biosens Bioelectron. 2011, 26: 3376-3381. 10.1016/j.bios.2010.12.035. View ArticlePubMedGoogle Scholar
- Su XL, Li Y: A QCM immunosensor for Salmonella detection with simultaneous measurements of resonant frequency and motional resistance. Biosens Bioelectron. 2005, 21: 840-848. 10.1016/j.bios.2005.01.021. View ArticlePubMedGoogle Scholar
- Cheng HY, Lai LJ, Ko FH: Rapid and sensitive detection of rare cancer cells by the coupling of immunomagnetic nanoparticle separation with ELISA analysis. Int J Nanomedicine. 2012, 7: 2967-2973. PubMed CentralView ArticlePubMedGoogle Scholar
- Zhou F, Wang M, Yuan L, Cheng Z, Wu Z, Chen H: Sensitive sandwich ELISA based on a gold nanoparticle layer for cancer detection. Analyst. 2012, 137: 1779-1784. 10.1039/c2an16257a. View ArticlePubMedGoogle Scholar
- Tamer U, Cetin D, Suludere Z, Boyaci IH, Temiz HT, Yegenoglu H, Daniel P, Dincer I, Elerman Y: Gold-coated iron composite nanospheres targeted the detection of Escherichia coli. Int J Mol Sci. 2013, 14: 6223-6240. 10.3390/ijms14036223. PubMed CentralView ArticlePubMedGoogle Scholar
- Li F, Zhou R, Zhao K, Chen H, Hu Y: Magnetic beads-based electrochemical immunosensor for detection of pseudorabies virus antibody in swine serum. Talanta. 2011, 87: 302-306. View ArticlePubMedGoogle Scholar
- Wang J, Moore J, Laulhe S, Nantz M, Achilefu S, Kang KA: Fluorophore-gold nanoparticle complex for sensitive optical biosensing and imaging. Nanotechnology. 2012, 23: 095501-10.1088/0957-4484/23/9/095501. View ArticlePubMedGoogle Scholar
- Gan N, Jin H, Li T, Zheng L: Fe(3)O(4)/Au magnetic nanoparticle amplification strategies for ultrasensitive electrochemical immunoassay of alfa-fetoprotein. Int J Nanomedicine. 2011, 6: 3259-3269. PubMed CentralView ArticlePubMedGoogle Scholar
- Huang SH, Liao MH, Chen DH: Direct binding and characterization of lipase onto magnetic nanoparticles. Biotechnol Prog. 2003, 19: 1095-1100. 10.1021/bp025587v. View ArticlePubMedGoogle Scholar
- Pandey P, Singh SP, Arya SK, Gupta V, Datta M, Singh S, Malhotra BD: Application of thiolated gold nanoparticles for the enhancement of glucose oxidase activity. Langmuir. 2007, 23: 3333-3337. 10.1021/la062901c. View ArticlePubMedGoogle Scholar
- Cudjoe KS, Thorsen LI, Sorensen T, Reseland J, Olsvik O, Granum PE: Detection of Clostridium perfringens type A enterotoxin in faecal and food samples using immunomagnetic separation (IMS)-ELISA. Int J Food Microbiol. 1991, 12: 313-321. 10.1016/0168-1605(91)90145-F. View ArticlePubMedGoogle Scholar
- Bai L, Liu X, Fu P, Guo Y: Serotyping and virulence genes of suspected Escherichia coli O157 strains in food from 2005 to 2007. Wei Sheng Yan Jiu. 2010, 39: 335-338. PubMedGoogle Scholar
- Ministry of Health P.R. China: Microbiological examination of food hygiene-examination of Escherichia coli O157:H7/NM. Standardization Administration PR China. 2008, GB/T 4789.36-2008 Google Scholar
- Duan HL, Shen ZQ, Wang XW, Chao FH, Li JW: Preparation of immunomagnetic iron-dextran nanoparticles and application in rapid isolation of E. coli O157:H7 from foods. World J Gastroenterol. 2005, 11: 3660-3664. PubMed CentralView ArticlePubMedGoogle Scholar
- Ambrosi A, Castaneda MT, Killard AJ, Smyth MR, Alegret S, Merkoci A: Double-codified gold nanolabels for enhanced immunoanalysis. Anal Chem. 2007, 79: 5232-5240. 10.1021/ac070357m. View ArticlePubMedGoogle Scholar
- Kong XL, Qiao FY, Qi H, Li FR: One-step preparation of antibody and oligonucleotide dual-labeled gold nanoparticle bio-probes and their properties. Biotechnol Lett. 2008, 30: 2071-2077. 10.1007/s10529-008-9802-6. View ArticlePubMedGoogle Scholar
- Nam JM, Thaxton CS, Mirkin CA: Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003, 301: 1884-1886. 10.1126/science.1088755. View ArticlePubMedGoogle Scholar
- Sherwani S, Farleigh L, Agarwal N, Loveless S, Robertson N, Hadaschik E, Schnitzler P, Bugert JJ: Seroprevalence of Molluscum contagiosum virus in German and UK populations. PLoS One. 2014, 9: e88734-10.1371/journal.pone.0088734. PubMed CentralView ArticlePubMedGoogle Scholar
- Cutler JI, Zheng D, Xu X, Giljohann DA, Mirkin CA: Polyvalent oligonucleotide iron oxide nanoparticle “click” conjugates. Nano Lett. 2010, 10: 1477-1480. 10.1021/nl100477m. PubMed CentralView ArticlePubMedGoogle Scholar
- Kumar A, Jena PK, Behera S, Lockey RF, Mohapatra S: Multifunctional magnetic nanoparticles for targeted delivery. Nanomedicine. 2010, 6: 64-69. 10.1016/j.nano.2009.04.002. PubMed CentralView ArticlePubMedGoogle Scholar
- Liu M, Jia C, Huang Y, Lou X, Yao S, Jin Q, Zhao J, Xiang J: Highly sensitive protein detection using enzyme-labeled gold nanoparticle probes. Analyst. 2010, 135: 327-331. 10.1039/b916629g. View ArticlePubMedGoogle Scholar
- Almeida C, Sousa JM, Rocha R, Cerqueira L, Fanning S, Azevedo NF, Vieira MJ: Detection of Escherichia coli O157 by peptide nucleic acid fluorescence in situ hybridization (PNA-FISH) and comparison to a standard culture method. Appl Environ Microbiol. 2013, 79: 6293-6300. 10.1128/AEM.01009-13. PubMed CentralView ArticlePubMedGoogle Scholar
- Chan KY, Ye WW, Zhang Y, Xiao LD, Leung PH, Li Y, Yang M: Ultrasensitive detection of E. coli O157:H7 with biofunctional magnetic bead concentration via nanoporous membrane based electrochemical immunosensor. Biosens Bioelectron. 2013, 41: 532-537. View ArticlePubMedGoogle Scholar
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/4.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Papers, Zotero, Reference Manager, RefWorks (.RIS)
EndNote (.ENW)
Mendeley, JabRef (.BIB)
Share this article
- Share on Twitter
- Share on Facebook
- Share on LinkedIn
- Share on Weibo
- Share on Google Plus
- Share on Reddit
See updates
Other Actions
Gut Pathogens
Contact us
- Editorial email: krishna.vairamani@springer.com
- Support email: info@biomedcentral.com
Follow BMC :
© 2018 BioMed Central Ltd unless otherwise stated. Part of Springer Nature.
Vergleichende Untersuchungen über die Plasmidverteilung bei Escherichia coli (E. coli)-Stämmen von gesunden und durchfallkranken Hunden sowie von Hundebesitzern
Dr. Ulrike H. Wastlhuber ,
- Institut für Pathologie und Rechtsmedizin der Universität Ulm (Direktor: Prof. Dr. O. Haferkamp)
- Institut für Medizinische Mikrobiologie, Infektions- und Seuchenmedizin der Universität München (Vorstand: Prof. Dr. Dr. h. c. mult. A. Mayr)
- Institut für Medizinische Mikrobiologie, Infektions- und Seuchenmedizin der Universität München (Vorstand: Prof. Dr. Dr. h. c. mult. A. Mayr)
- Institut für Medizinische Mikrobiologie, Infektions- und Seuchenmedizin der Universität München (Vorstand: Prof. Dr. Dr. h. c. mult. A. Mayr)
- First published: 12 January 1988 Full publication history
- DOI: 10.1111/j.1439-0450.1988.tb00491.x View/save citation
- Cited by (CrossRef): 0 articles Check for updates
Institut für Med. Mikrobiologie, Inf.- u. Seuchenmedizin, Veterinärstraße 13, D-8000 München 22
Summary
Comparative investigations on the distribution of plasmids in E. coli strains from dogs with diarrhoea, healthy dogs and their owners
A total of 614 E. coli-strains taken from the faeces samples of healthy dogs, dogs with diarrhoea and their owners were examined for plasmid content. The plasmid patterns were shown by agarose electrophoresis.
Around 20% of the E. coli strains from the faeces samples were free of plasmid. The remaining 80% of the strains had up to 13 plasmids displaying 11 different distribution patterns altogether. Typical plasmid patterns for E. coli strains from healthy dogs, dogs with diarrhoea and their owners could not be demonstrated.
ETEC-strains were only isolated from sick dogs. These strains all contained plasmids. Hemolysin-forming E. coli strains appeared in three of the groups examined and either contained plasmids or proved plasmid-free, as with the mucoid-growing strains.
On comparison of the E. coli strains from faeces samples of dogs and their owners, it was possible in the case of one family to isolate strains with identical plasmid patterns from the dog and the child. In 4 other families, individual plasmids of the E. coli strain from faeces samples of the dog matched those of the family members.
Zusammenfassung
Insgesamt 614 E. coli-Stämme aus Fäzesproben von gesunden und durchfallkranken Hunden sowie von Hundebesitzern wurden auf ihren Plasmidgehalt untersucht. Die Darstellung der Plasmidmuster erfolgte mittels Agarosegelektrophorese.
Rund 20% der E. coli-Stämme aus den Kotproben der Hunde und Besitzer waren plasmidfrei. Die übrigen 80% der Stämme hatte bis zu 13 Plasmide, die ingesamt 11 verschiedene Verteilungsmuster aufwiesen. Typische Plasmidmuster für E. coli-Stämme aus gesunden und durchfallkranken Hunden sowie Hundebesitzern konnten nicht nachgewiesen werden.
ETEC-Stämme wurden nur aus kranken Hunden isoliert. Alle diese Stämme besaßen Plasmide. Hämolysinbildende E. coli-Stämme traten in allen drei untersuchten Gruppen auf und waren sowohl plasmidfrei als auch plasmidhaltig, ebenso die mucoid wachsenden Stämme.
Beim Vergleich der E. coli-Stämme aus Kotproben von Hunden und den Besitzern konnten in einer Familie beim Kind und beim Hund Stämme mit identischen Plasmidmustern isoliert werden. In 4 anderen Familien stimmten einzelne Plasmide der E. coli-Stämme aus Kotproben des Hundes mit denen der Familienmitglieder überein.
Article Information
Format Available
© 1988 Blackwell Verlag GmbH
- E. coli;
- plasmid pattern;
- dog;
- diarrhoea;
- owner
Publication History
- Issue online: 13 May 2010
- Version of record online: 13 May 2010
- Received for publication 23. Februar 1988
Related content
Articles related to the one you are viewing
Citing Literature
- Number of times cited : 0
Copyright © 1999 - 2018 John Wiley & Sons, Inc. All Rights Reserved
Holistic Treatment Of Chronic Vaginitis
Our Golden / Poodle mix has had chronic vaginitis since she was 10 weeks old (now 15 months) and nothing seems to work. Antibiotics are only temporary relief and we do not want to give her these any more. We have a holistic vet and she has been on San Ren Tang, which does clear things up, however she gets ear infections every time we put her on this for a week or so. When we stop the San Re Tang the vaginitis returns. She gets a probiotic 2 x per day, feed 100% raw, treats are all natural and dehydrated with no additives. We are at a loss at this point on how to treat. Any recommendations?
 Dear Lynn,
Dear Lynn,
You are doing a number of things that are often helpful for female dogs with vaginitis.
Vaginitis, and indeed many skin inflammations, can be related to food sensitivities or the inflammatory substances present in many processed foods, so a raw or minimally processed diet is ideal.
Probiotics have been shown to dramatically help women with vaginitis, and this is true of female canines as well. All too often, however, probiotics do not have very many active organisms. Be sure to choose a probiotic that is fresh and has a large number of active organisms. One of my favourite products is Culturelle. Your girl would get as much as an adult human.
To cure your dog’s vaginitis, rather than just temporarily suppressing the symptoms, we need to understand vaginitis, and then address the cause of the problem. Vaginitis is an inflammation of the vagina. Puppy vaginitis is excessive production of sticky white to yellow mucus in female pups; it can occur as early as six weeks of age, and generally resolves on its own with puberty. The discharge may not bother a pup at all, but some pups lick excessively, causing inflammation or dermatitis of the skin around the vulva. Puppy vaginitis can be confused with urinary tract infections or anatomical malformations, so vets need to check for these before diagnosing puppy vaginitis. True puppy vaginitis is a benign condition, and should not be treated with antibiotics or douches. Puppy vaginitis is part of the maturation process of the reproductive tract. Most baby bitches need no treatment at all. For cleanliness, it may be desirable to gently wipe off excessive discharge once or twice daily with an unscented baby wipe. If there is a lot of moisture, cornstarch (no talc) baby powder will prevent chafing and irritation. Aggressive treatment of benign puppy vaginitis with antibiotics unbalances the normal bacterial population of the skin, which creates conditions for a fungal or bacterial infection of the area.
Most simple inflammations of the vagina and vulvar area can be treated with gentle topical treatments. First, we must address obstacles to cure.
Does your dog have a urinary tract infection (UTI) or crystals in the urine at this time? A UTI will predispose any individual to vulvar inflammation, and the UTI should be addressed first. Constitutional homeopathic treatment is ideal for this. Crystals in the urine may be a result of inadequate fluid intake, and increased water consumption is always helpful. Water can be added directly to ground raw diets, or dogs can be offered low sodium meat broth. A repeated tendency to form crystals can be addressed with constitutional homeopathic treatment or herbal treatment.
Was your female spayed at an early age? Many females who are spayed before puberty have very small external genitalia which lie in a recessed position. These immature genitalia trap moisture after the dog urinates, resulting in inflammation from chafing of the moist surfaces. I see many spayed bitches who have received multiple courses of antibiotics for vulvar skin inflammation, often diagnosed as vaginitis. In truth, most of these girls have vulvar dermatitis because of their recessed vulva. It is simple to institute a twice daily gentle and thorough cleaning regimen with unscented baby wipes, followed by gentle powdering with cornstarch (no talc) baby powder. The deep creases along the vulva must be gently cleansed and powdered until the inflammation subsides. After that, it is often sufficient to powder once daily.
Is your female dog overweight? Obese animals have redundant folds of fat and tissue which can retain moisture, block air flow, and cause moist dermatitis. Weight control is essential, combined with proper hygiene of the perivulvar tissue as discussed above.
When any patient develops a new problem when on a medication, it most often means that medication is not well suited to that individual. Exception to this are aggravations from homeopathic remedies, and detox reactions; in such cases a practitioner will adjust the dose of the medication to avoid unnecessary suffering by the patient. It is not wise to repeatedly stimulate an individual’s body to have these unpleasant reactions. As your Golden / Poodle develops ear infections every time she is on San Ren Tang, I would suggest that you discuss this with your Traditional Chinese Medicine (TCM) practitioner. There are a number of other TCM combinations (possibly Si Miao San or Yu Dai Wan) which could be helpful if herbal treatment of your girl’s vaginitis is necessary. Please do not self-medicate with Chinese herbal formulas without the advice of a TCM practitioner.
About the Author Dr Sara Chapman, DVM, MRCVS, VetMFHom
Dr Sara Chapman graduated from Ohio State University in 1985. In 1995, she became interested in homeopathy after her own chronically lame horse responded dramatically to it. In May 2002, and passed the exams to become a Veterinary Member of the Faculty of Homeopathy (VetMFHom – UK) later that year. During 2007-2008, Dr Chapman took the International Association of Veterinary Acupuncture course in acupuncture and Traditional Chinese Medicine in San Antonio, and passed her qualifying exam. Dr Chapman is a member of the Academy of Veterinary Homeopathy along with many other respected holistic organizations.
You might also like .
What To Do If Your Dog Eats Chocolate … And 7 Other Issues
The Benefits of Cell Salts
5 Healing Crystals to Help Your Dog
How To Use Healing Clay For Dogs
Is Aloe Poisonous For Dogs?
3 Natural Solutions for Prostate Problems
The Benefits Of Mullein For Dogs
A Natural Approach to Managing Degenerative Myelopathy
The Healthy Pet Tool Box: To Help Boost Your Dog’s Health & Longevity
Anise, Fennel, Licorice – What’s the Difference?
Tumor, But No Surgery? What Will People Think??
Nature vs Nurture: Epigenetics And Your Dog’s Health
Untersuchungen über die Coli-Infektion als Sterilitätsursache beim Hund
W. Baier,
- Gynäkologischen und Ambulatorischen Tierklinik der Universität München, Vorstand: Prof. Dr. W. Baier
- Tierhygienischen Institut der Universität München, Vorstand: Prof. Dr. A. Meyn
- Gynäkologischen und Ambulatorischen Tierklinik der Universität München, Vorstand: Prof. Dr. W. Baier
- Tierhygienischen Institut der Universität München, Vorstand: Prof. Dr. A. Meyn
J. Taxacher
- Gynäkologischen und Ambulatorischen Tierklinik der Universität München, Vorstand: Prof. Dr. W. Baier
- Tierhygienischen Institut der Universität München, Vorstand: Prof. Dr. A. Meyn
- First published: November 1956 Full publication history
- DOI: 10.1111/j.1439-0442.1956.tb00171.x View/save citation
- Cited by (CrossRef): 0 articles Check for updates
Zusammenfassung
- 1 Coli-Infektionen können beim Hund zu spezifischen Erkrankungen der Geschlechtsorgane führen. Die natürliche Infektion männlicher Tiere und die Übertragung auf weibliche Tiere wird beschrieben.
- 2 Die Identität der vom rüden und den hündinnen isolierten Coli-Stämme wird durch serologische Kreuzreaktionen überprüft.
- 3 Gesunde Samenzellen werden durch toxische Coli-Keime agglutiniert oder in ihrer Vitalität geschwächt.
- 4 Die Infektionen sind bei noch nicht aufgetretenen Parenchymschäden durch gezielte antibiotische Therapie, speziell durch Aureomycin, bekämpfbar, besonders, wenn zugleich die physiologische Darmflora gesichert oder wiederhergestellt wird.Coli-Infektionen können zu reversibler und irreversibler Sterilität führen.
Studies on infection with E. coli as a cause of sterility in the dog
I. Bacterial infections of the male sex organs, especially the testis
- 1 Infection with E. coli can lead in the dog to specific diseases of the sex organs. Natural infection of males and its transmission to females are described.
- 2 Strains isolated from dogs and bitches were identified serologically.
- 3 Healthy germ cells were agglutinated by toxic E. coli or their vitality was lessened.
- 4 Infections which have not yet produced parenchymatous injury can be controlled by suitable antibiotics, especially aureomycin, and particularly if, at the same time, the physiological intestinal flora is safeguarded or re-established.Infections with E. coli can lead to reversible or irreversible sterility.
Recherches sur la colibacillose du chien, cause possible de stérilité
- 1 Des infections colibacillaires peuvent être la cause chez le chien de maladies spécifiques des organes génitaux. Les auteurs décrivent l'infection naturelle des animaux mâles et la contamination des animaux femelles.
- 2 L'identité des souches de colibacilles isolées chez le chien mâle et chez les chiennes fut confirmée par des réactions sérologiques croisées.
- 3 Les spermatozoïdes sains sont agglutinés par les colibacilles toxiques ou perdent de leur vitalité.
- 4 Les infections peuvent être combattues, tant que le parenchyme n'est pas lésé, par un traitement rationnel aux antibiotiques, notamment par l'auréomycine, surtout si l'on prend soin des protéger ou de renouveler la flore intestinale normale.Les infections colibacillaires peuvent entraîner une stérilité réversible ou irréversible.
Estudios sobre la infección por colis como causa de esterilidad en la perra
- 1 En el perro, las infecciones por colis pueden producir lesiones específicas en los órganos genitales. Se describe la infección natural de los machos y la transmisión a las hembras.
- 2 Con ayuda de reacciones serológicas cruzadas se comprobó la identidad de las cepas coli aisladas en perros y perras.
- 3 Las células seminales sanas son aglutinadas por gérmenes coli tóxicos o debilitadas en su vitalidad.
- 4 Cuando todavía no han surgido las lesiones en el parénquima, las infecciones pueden combatirse mediante la terapéutica antibiótica dirigida, en especial cuando se afirma o restablece la flora intestinal fisiológica.Las infecciones por colis pueden dar origen a una esterilidad reversible o irreversible.
Article Information
Format Available
© 1956 Blackwell Verlag GmbH
Publication History
- Issue online: 13 May 2010
- Version of record online: 13 May 2010
Related content
Articles related to the one you are viewing
Citing Literature
- Number of times cited : 0
Copyright © 1999 - 2018 John Wiley & Sons, Inc. All Rights Reserved
Комментариев нет:
Отправить комментарий